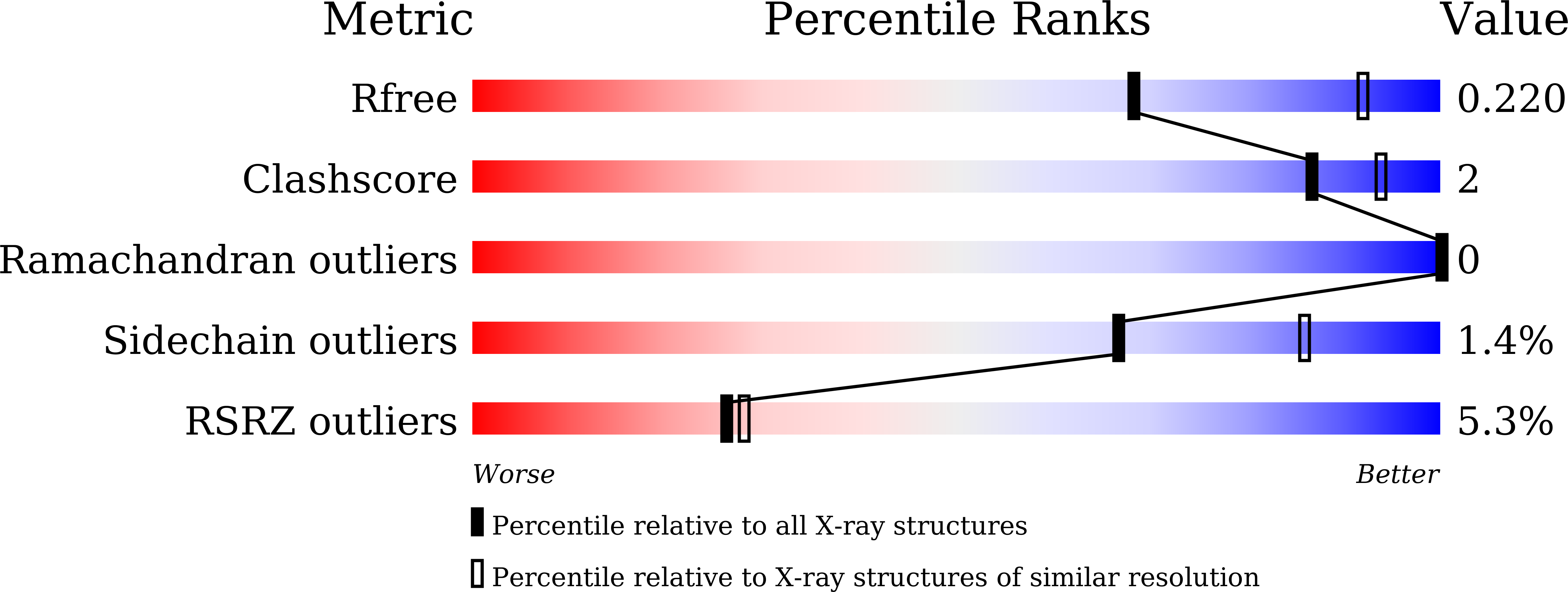
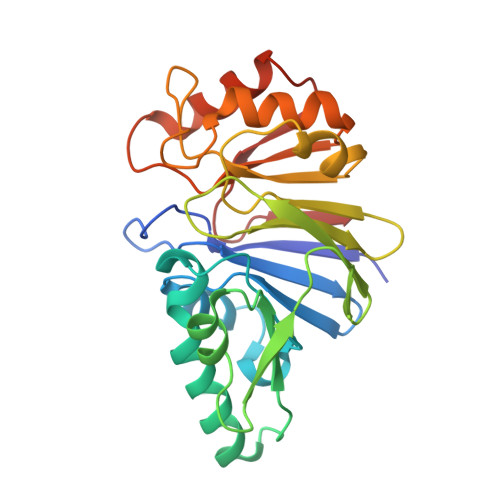
Structural and biochemical analyses of the metallo-beta-lactamase fold protein YhfI from Bacillus subtilis.
Na, H.W., Namgung, B., Song, W.S., Yoon, S.I.(2019) Biochem Biophys Res Commun 519: 35-40
- PubMed: 31481231
- DOI: https://doi.org/10.1016/j.bbrc.2019.08.106
- Primary Citation of Related Structures:
6KNS, 6KNT - PubMed Abstract:
Metallo-β-lactamase (MBL) fold proteins play critical roles in diverse biological processes, such as DNA repair, RNA processing, detoxification, and metabolism. Although MBL fold proteins share a metal-bound αββα structure, they are highly heterogeneous in metal type, metal coordination, and oligomerization and exhibit different catalytic functions. Bacillus subtilis contains the yhfI gene, which is predicted to encode an MBL fold protein. To reveal the structural and functional features of YhfI, we determined two crystal structures of YhfI and biochemically characterized the catalytic activity of YhfI. YhfI forms an α-helix-decorated β-sandwich structure and assembles into a dimer using highly conserved residues. Each YhfI chain simultaneously interacts with two metal ions, which are coordinated by histidine and aspartate residues that are strictly conserved in YhfI orthologs. A comparative analysis of YhfI and its homologous structures suggests that YhfI would function as a phosphodiesterase. Indeed, YhfI drove the phosphodiesterase reaction and showed high catalytic activity at pH 8.0-9.5 in the presence of manganese. Moreover, we propose that the active site of YhfI is located at a metal-containing pocket generated between the two subunits of a YhfI dimer.
Organizational Affiliation:
Division of Biomedical Convergence, College of Biomedical Science, Kangwon National University, Chuncheon, 24341, Republic of Korea.