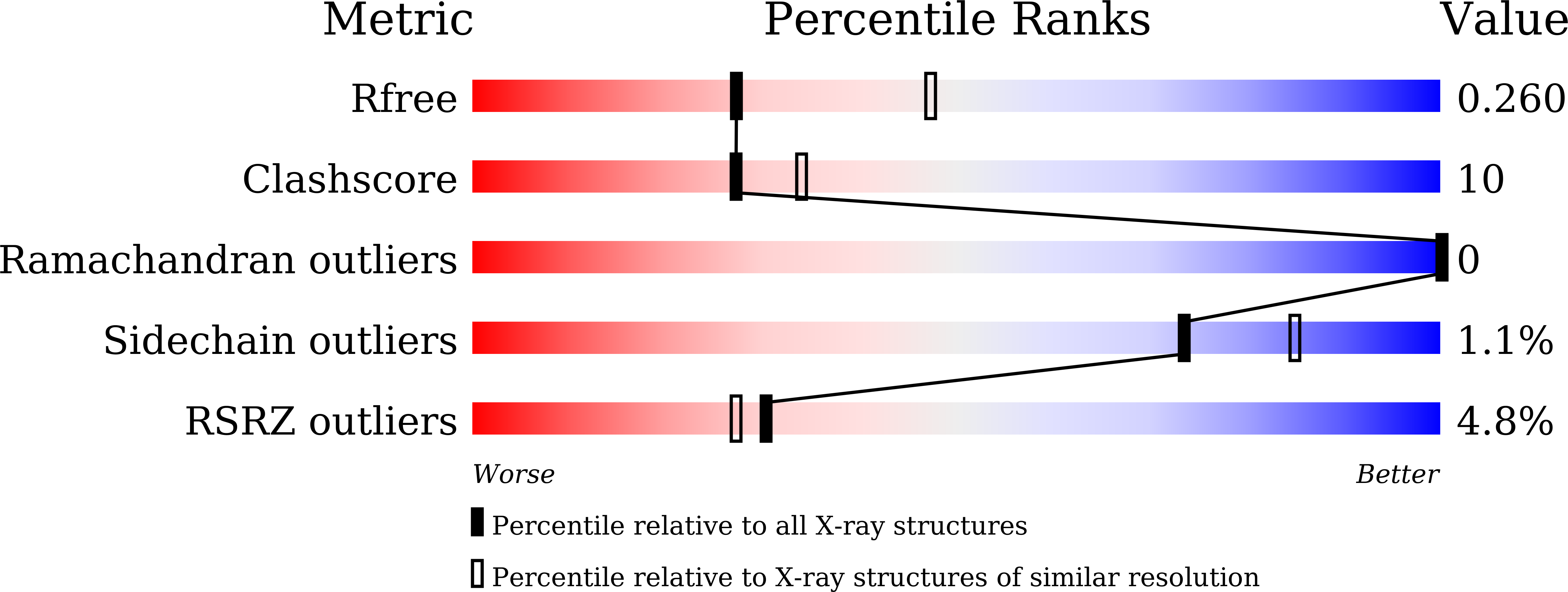
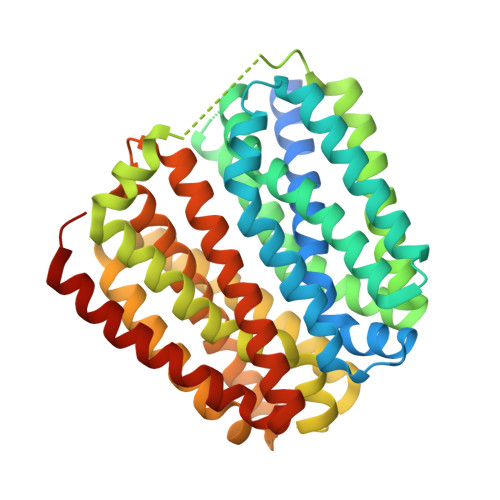
Visualizing the nonlinear changes of a drug-proton antiporter from inward-open to occluded state.
Xiao, Q., Sun, B., Zhou, Y., Wang, C., Guo, L., He, J., Deng, D.(2021) Biochem Biophys Res Commun 534: 272-278
- PubMed: 33280821
- DOI: https://doi.org/10.1016/j.bbrc.2020.11.096
- Primary Citation of Related Structures:
6KKI, 6KKJ, 6KKK, 6KKL - PubMed Abstract:
Drug-proton antiporters (DHA) play an important role in multi-drug resistance, utilizing the proton-motive force to drive the expulsion of toxic molecules, including antibiotics and drugs. DHA transporters belong to the major facilitator superfamily (MFS), members of which deliver substrates by utilizing the alternating access model of transport. However, the transport process is still elusive. Here, we report the structures of SotB, a member of DHA1 family (TCDB: 2.A.1.2) from Escherichia coli. Four crystal structures of SotB were captured in different conformations, including substrate-bound occluded, inward-facing, and inward-open states. Comparisons between the four structures reveal nonlinear rigid-body movements of alternating access during the state transition from inward-open to occluded conformation. This work not only reveals the conformational dynamics of SotB but also deepens our understanding of the alternating access mechanism of MFS transporters.
Organizational Affiliation:
From the Division of Obstetrics, Key Laboratory of Birth Defects and Related Disease of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second Hospital, Sichuan University, Chengdu, 610041, China.