Structure of a hypothetical protease
Liao, S., Gao, J., Xu, C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Small vasohibin-binding protein | 49 | Homo sapiens | Mutation(s): 0 Gene Names: SVBP, CCDC23 | 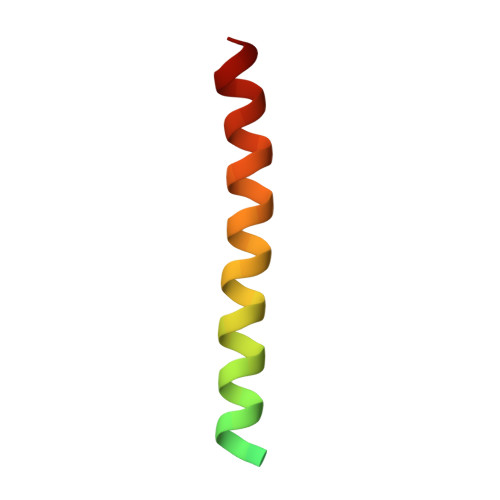 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8N300 (Homo sapiens) Explore Q8N300 Go to UniProtKB: Q8N300 | |||||
PHAROS: Q8N300 GTEx: ENSG00000177868 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8N300 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tubulinyl-Tyr carboxypeptidase 1 | 238 | Homo sapiens | Mutation(s): 0 Gene Names: VASH1, KIAA1036, VASH EC: 3.4.17.17 | 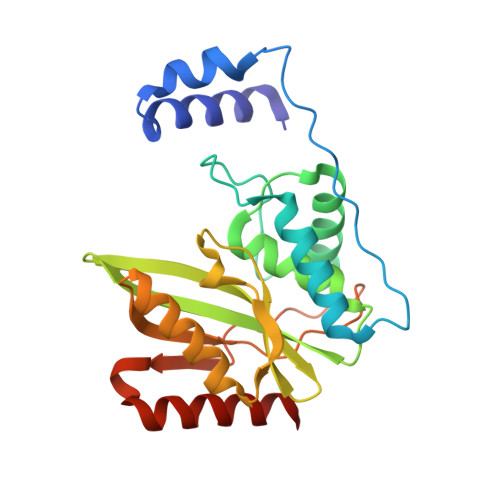 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q7L8A9 (Homo sapiens) Explore Q7L8A9 Go to UniProtKB: Q7L8A9 | |||||
PHAROS: Q7L8A9 GTEx: ENSG00000071246 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7L8A9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 8-mer peptide | 8 | Homo sapiens | Mutation(s): 0 EC: 3.6.5 | 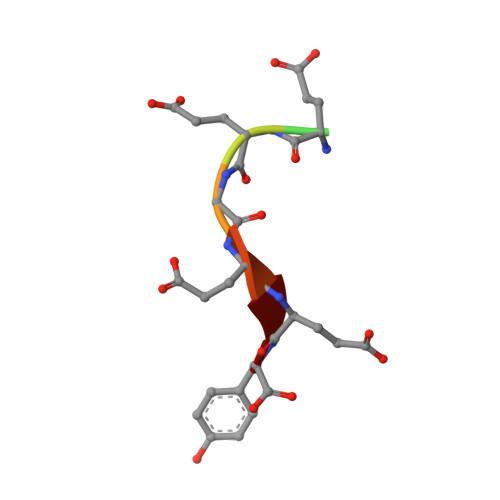 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P68363 (Homo sapiens) Explore P68363 Go to UniProtKB: P68363 | |||||
PHAROS: P68363 GTEx: ENSG00000123416 | |||||
Entity Groups | |||||
| UniProt Group | P68363 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 69.641 | α = 90 |
| b = 127.36 | β = 90 |
| c = 44.31 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China | China | 31500601 |
| National Natural Science Foundation of China | China | 31570737 |
| National Natural Science Foundation of China | China | 31770806 |