Acquired Removability of Aspartic Protease Inhibitors by Direct Biotinylation.
Hidaka, K., Adachi, M., Tsuda, Y.(2019) Bioconjug Chem 30: 1979-1985
- PubMed: 30990716
- DOI: https://doi.org/10.1021/acs.bioconjchem.9b00195
- Primary Citation of Related Structures:
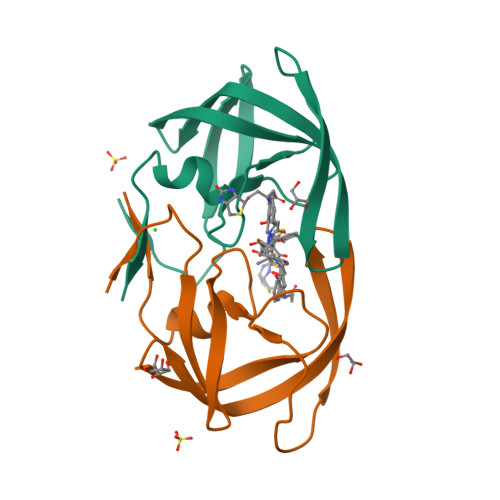
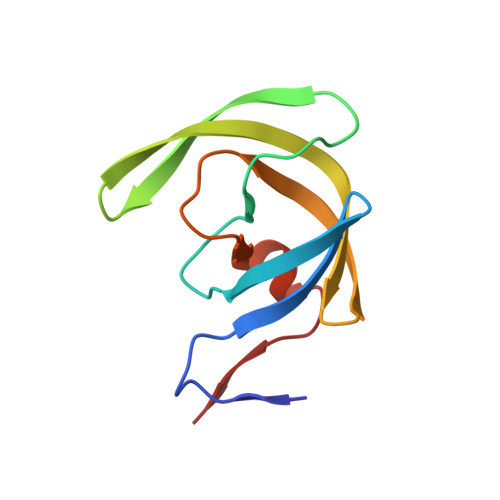
6IXD - PubMed Abstract:
Protease inhibitors are used as both research tools and therapeutics. Many of these inhibitors consist of substrate amino acid sequence-derived structure with a transition state mimic to interact with the active site of the protease, suppressing enzymatic activity. However, once they bind, macrodilution or protein denaturation is required to remove them, limiting their usage. In this study, we describe a removable protease inhibitor, which is a directly biotinylated analogue to control the activities of HIV-1 protease and human cathepsin D. In the substrate cleavage assay, we observed that the nanomolar inhibitory activities were lost upon the addition of streptavidin, while the enzymatic activities sufficiently recovered. HIV-1 protease mixed with the removable inhibitor, avoiding autolysis, was still active to be detected by adding streptavidin after one year at room temperature. We also observed that the inhibitor was an effective eluent for the simple detection of the activity of proteases purified from human serum and cells. These results demonstrate that direct biotinylation of protease inhibitors could be a novel method for controlling the enzymatic activity from OFF to ON. We proposed the phenomenon that binding equilibrium of inhibitor was shifted from protease to streptavidin with higher affinity, named "inhibitor stripping action by affinity competition", or ISAAC. We anticipate that ISAAC could be applicable for preservatives of proteases and activity-based diagnosis of protease related diseases. Furthermore, removable inhibitor to be designed for targeted proteases changing the inhibitor structure may elucidate enzymatic activity in intrinsic form with natural modifications from various biological samples.
Organizational Affiliation:
Institute for Quantum Life Science , National Institutes for Quantum and Radiological Science and Technology , Tokai, Ibaraki , 319-1106 , Japan.