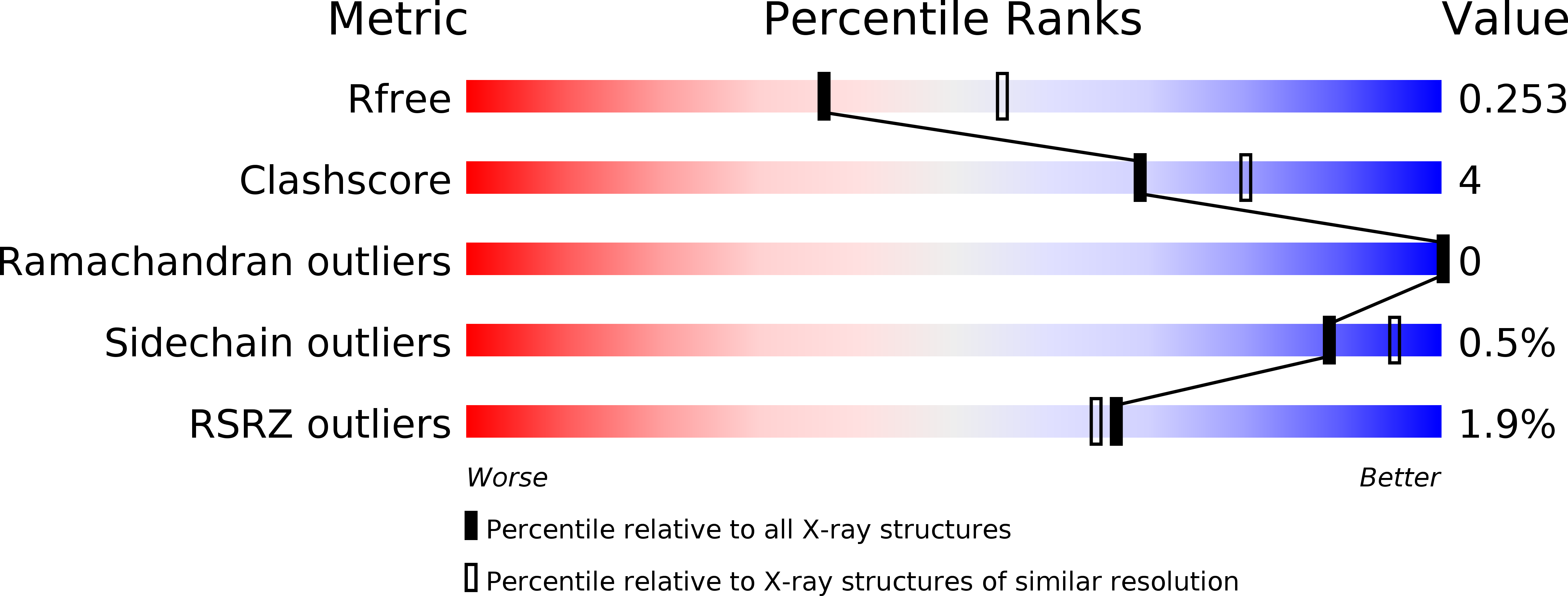
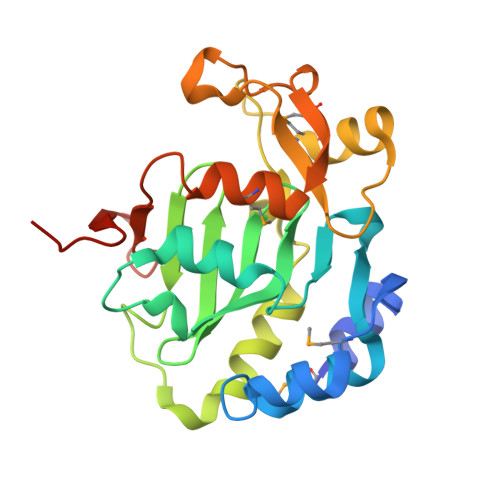
Crystal structure of the type VI immunity protein Tdi1 (Atu4351) from Agrobacterium tumefaciens.
Shi, L., Gao, Z., Zhang, T., Zhang, H., Dong, Y.(2019) Acta Crystallogr F Struct Biol Commun 75: 153-158
- PubMed: 30839288
- DOI: https://doi.org/10.1107/S2053230X19000815
- Primary Citation of Related Structures:
6ITW - PubMed Abstract:
The type VI secretion system (T6SS) is a novel multiprotein needle-like apparatus that is distributed widely in Gram-negative bacteria. Bacteria harboring T6SSs inject various effectors into both eukaryotic and prokaryotic cells for interspecies competition or virulence-related processes. The toxicities of the effectors can be neutralized by their cognate immunity proteins. Tde1 (Atu4350)-Tdi1 (Atu4351) has recently been characterized as a T6SS effector-immunity pair in the soil bacterium Agrobacterium tumefaciens and the neutralization mechanism remains unknown. Here, the crystal structure of the immunity protein Tdi1 was determined at 2.40 Å resolution by the single-wavelength anomalous dispersion method. Structural analysis suggested that it is composed of a GAD-like domain and an inserted DUF1851 domain, and both domains show low structural similarities to known structures. There is a positive groove mainly located in the GAD-like domain that may be associated with nucleotide binding. The structure provides a basis for further study of the positive groove as a potential active site.
Organizational Affiliation:
School of Life Sciences, University of Science and Technology of China, Hefei 230027, People's Republic of China.