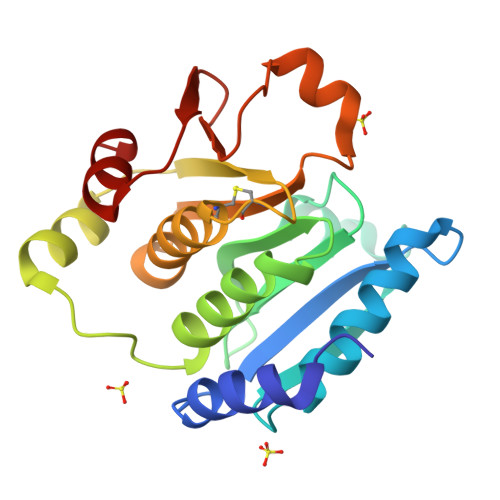
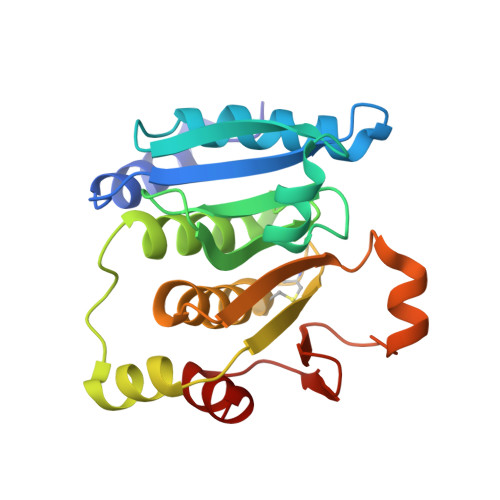
Effect of the additional cysteine 503 of vancomycin-resistant Enterococcus faecalis (V583) alkylhydroperoxide reductase subunit F (AhpF) and the mechanism of AhpF and subunit C assembling.
Toh, Y.K., Shin, J., Balakrishna, A.M., Kamariah, N., Gruber, A., Eisenhaber, F., Eisenhaber, B., Gruber, G.(2019) Free Radic Biol Med 138: 10-22
- PubMed: 31047989
- DOI: https://doi.org/10.1016/j.freeradbiomed.2019.04.036
- Primary Citation of Related Structures:
6IL7 - PubMed Abstract:
The vancomycin-resistant Enterococcus faecalis alkyl hydroperoxide reductase complex (AhpR) with its subunits AhpC (EfAhpC) and AhpF (EfAhpF) is of paramount importance to restore redox homeostasis. Therefore, knowledge about this defense system is essential to understand its antibiotic-resistance and survival in hosts. Recently, we described the crystallographic structures of EfAhpC, the two-fold thioredoxin-like domain of EfAhpF, the novel phenomenon of swapping of the catalytic domains of EfAhpF as well as the unique linker length, connecting the catalytically active N-and C-terminal domains of EfAhpF. Here, using mutagenesis and enzymatic studies, we reveal the effect of an additional third cysteine (C503) in EfAhpF, which might optimize the functional adaptation of the E. faecalis enzyme under various physiological conditions. The crystal structure and solution NMR data of the engineered C503A mutant of the thioredoxin-like domain of EfAhpF were used to describe alterations in the environment of the additional cysteine residue during modulation of the redox-state. To glean insight into the epitope and mechanism of EfAhpF and -AhpC interaction as well as the electron transfer from the thioredoxin-like domain of EfAhpF to AhpC, NMR-titration experiments were performed, showing a coordinated disappearance of peaks in the thioredoxin-like domain of EfAhpF in the presence of full length EfAhpC, and indicating a stable EfAhpF-AhpC-complex. Combined with docking studies, the interacting residues of EfAhpF were identified and a mechanism of electron transfer of the EfAhpF donor to the electron acceptor EfAhpC is described.
Organizational Affiliation:
Bioinformatics Institute, Agency for Science, Technology and Research (A*STAR), 30 Biopolis Street, #07-01 Matrix, Singapore, 138671, Republic of Singapore.