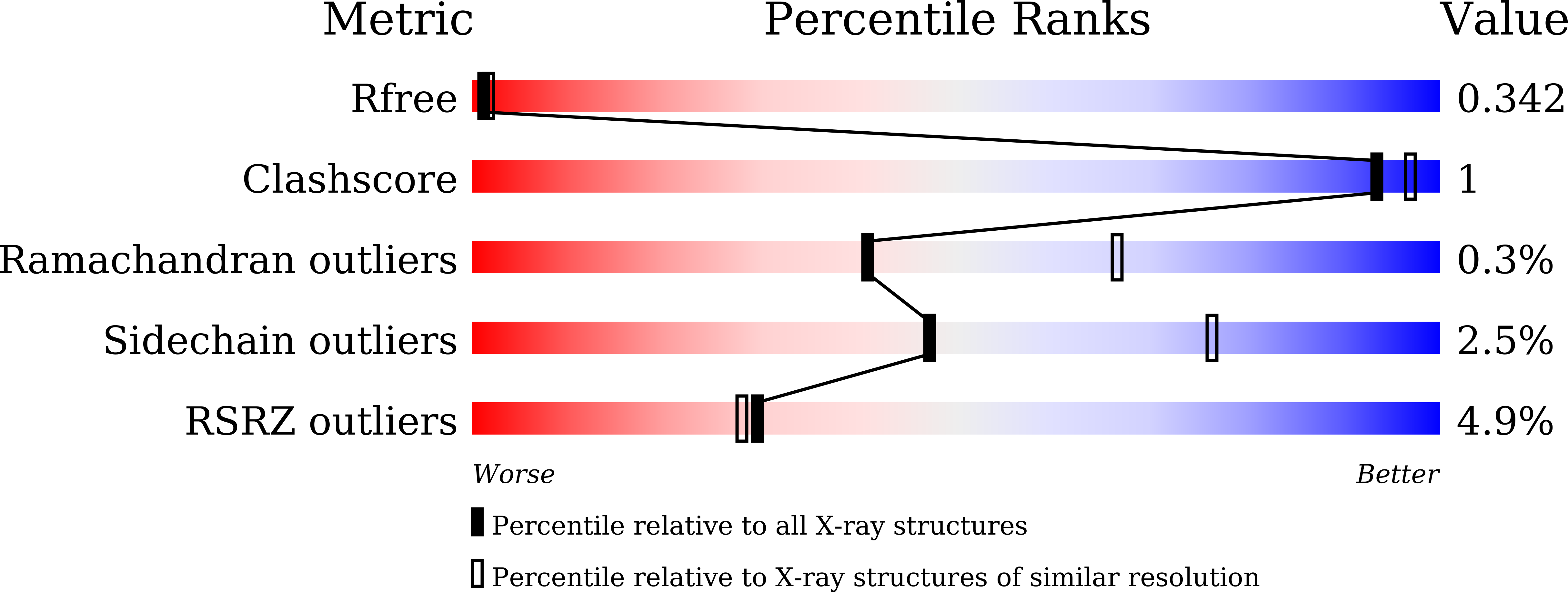
Validation of Phosphodiesterase-10 as a Novel Target for Pulmonary Arterial Hypertension via Highly Selective and Subnanomolar Inhibitors.
Huang, Y.Y., Yu, Y.F., Zhang, C., Chen, Y., Zhou, Q., Li, Z., Zhou, S., Li, Z., Guo, L., Wu, D., Wu, Y., Luo, H.B.(2019) J Med Chem 62: 3707-3721
- PubMed: 30888810
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00224
- Primary Citation of Related Structures:
6IJH, 6IJI - PubMed Abstract:
Pulmonary arterial hypertension (PAH) causes pathological increase in pulmonary vascular resistance, leading to right-heart failure and eventual death. Previously, phosphodiesterase-10 (PDE10) was reported to be a promising target for PAH based on the studies with a nonselective PDE inhibitor papaverine, but little progress has been made to confirm the practical application of PDE10 inhibitors. To validate whether PAH is ameliorated by PDE10 inhibition rather than other PDE isoforms, here we report an integrated strategy to discover highly selective PDE10 inhibitors as chemical probes. Structural optimization resulted in a PDE10 inhibitor 2b with subnanomolar affinity and good selectivity of >45 000-fold against other PDEs. The cocrystal structure of the PDE10-2b complex revealed an important H-bond interaction between 2b and Tyr693. Finally, compound 2b significantly decreased the arterial pressure in PAH rats and thus validated the potential of PDE10 as a novel anti-PAH target. These findings suggest that PDE10 inhibition may be a viable treatment option for PAH.
Organizational Affiliation:
School of Pharmaceutical Sciences , Sun Yat-sen University , Guangzhou 510006 , China.