Underlying molecular alterations in human dihydrolipoamide dehydrogenase deficiency revealed by structural analyses of disease-causing enzyme variants.
Szabo, E., Wilk, P., Nagy, B., Zambo, Z., Bui, D., Weichsel, A., Arjunan, P., Torocsik, B., Hubert, A., Furey, W., Montfort, W.R., Jordan, F., Weiss, M.S., Adam-Vizi, V., Ambrus, A.(2019) Hum Mol Genet 28: 3339-3354
- PubMed: 31334547
- DOI: https://doi.org/10.1093/hmg/ddz177
- Primary Citation of Related Structures:
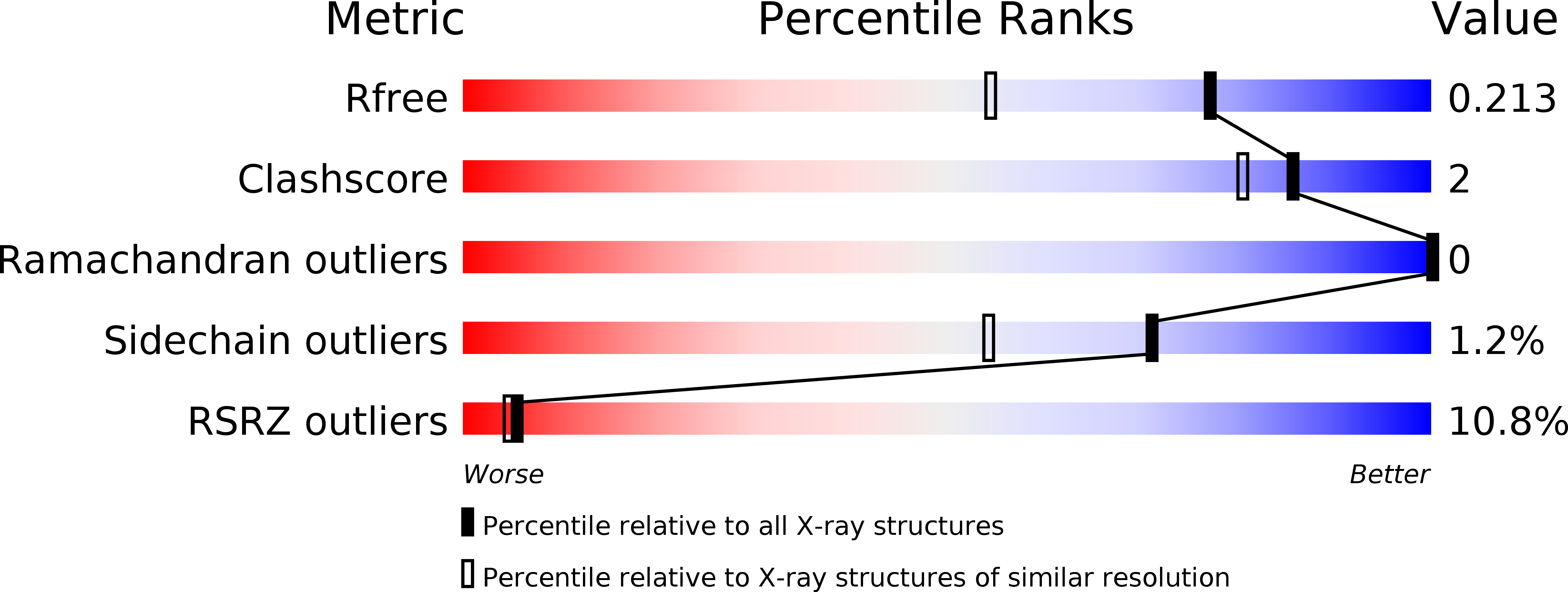
6I4P, 6I4Q, 6I4R, 6I4S, 6I4T, 6I4U, 6I4Z - PubMed Abstract:
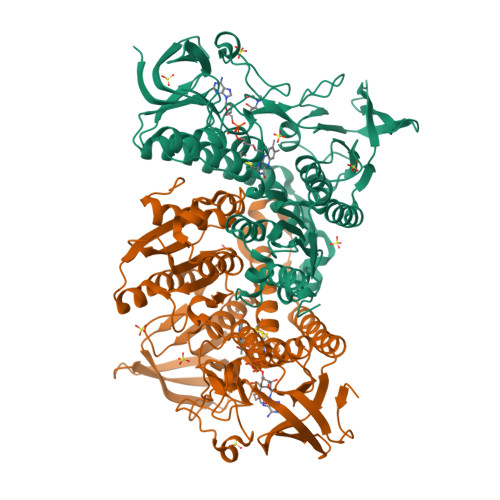
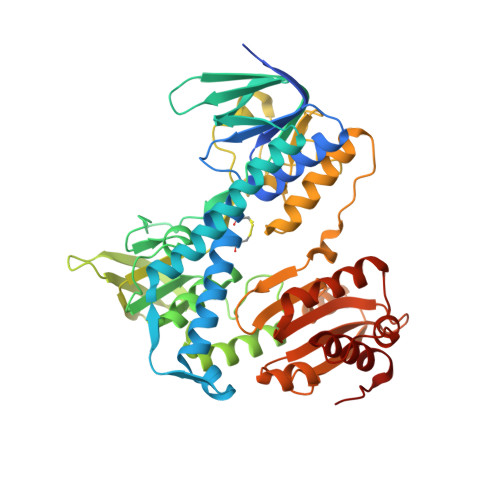
Human dihydrolipoamide dehydrogenase (hLADH, hE3) deficiency (OMIM# 246900) is an often prematurely lethal genetic disease usually caused by inactive or partially inactive hE3 variants. Here we report the crystal structure of wild-type hE3 at an unprecedented high resolution of 1.75 Å and the structures of six disease-causing hE3 variants at resolutions ranging from 1.44 to 2.34 Å. P453L proved to be the most deleterious substitution in structure as aberrations extensively compromised the active site. The most prevalent G194C-hE3 variant primarily exhibited structural alterations close to the substitution site, whereas the nearby cofactor-binding residues were left unperturbed. The G426E substitution mainly interfered with the local charge distribution introducing dynamics to the substitution site in the dimer interface; G194C and G426E both led to minor structural changes. The R460G, R447G and I445M substitutions all perturbed a solvent accessible channel, the so-called H+/H2O channel, leading to the active site. Molecular pathomechanisms of enhanced reactive oxygen species (ROS) generation and impaired binding to multienzyme complexes were also addressed according to the structural data for the relevant mutations. In summary, we present here for the first time a comprehensive study that links three-dimensional structures of disease-causing hE3 variants to residual hLADH activities, altered capacities for ROS generation, compromised affinities for multienzyme complexes and eventually clinical symptoms. Our results may serve as useful starting points for future therapeutic intervention approaches.
Organizational Affiliation:
Department of Medical Biochemistry, MTA-SE Laboratory for Neurobiochemistry, Semmelweis University, Budapest, 1094, Hungary.