Structure-Based in Silico Screening Identifies a Potent Ebolavirus Inhibitor from a Traditional Chinese Medicine Library.
Shaikh, F., Zhao, Y., Alvarez, L., Iliopoulou, M., Lohans, C., Schofield, C.J., Padilla-Parra, S., Siu, S.W.I., Fry, E.E., Ren, J., Stuart, D.I.(2019) J Med Chem 62: 2928-2937
- PubMed: 30785281
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01328
- Primary Citation of Related Structures:
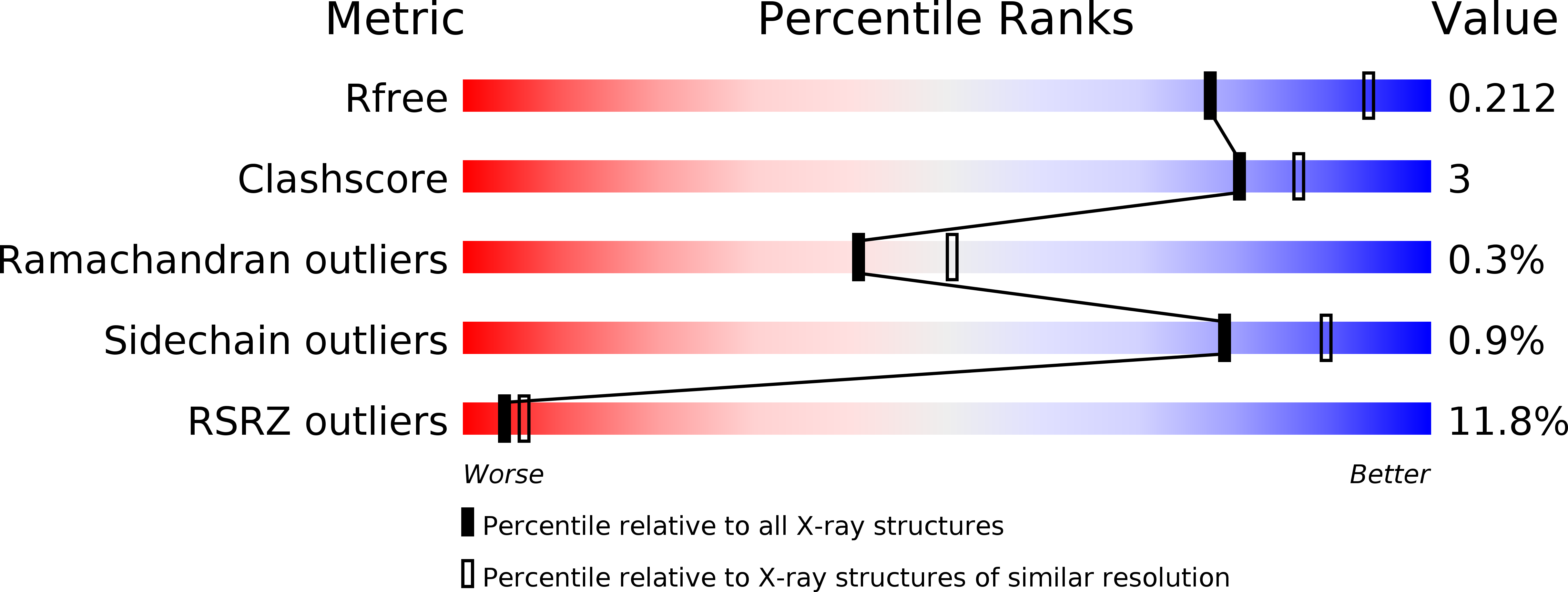
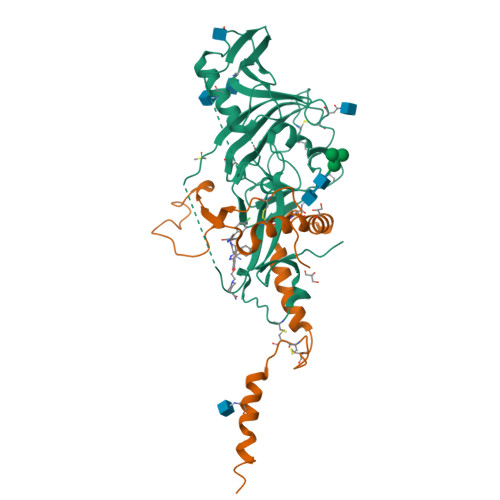
6HRO, 6HS4 - PubMed Abstract:
Potent Ebolavirus (EBOV) inhibitors will help to curtail outbreaks such as that which occurred in 2014-16 in West Africa. EBOV has on its surface a single glycoprotein (GP) critical for viral entry and membrane fusion. Recent high-resolution complexes of EBOV GP with a variety of approved drugs revealed that binding to a common cavity prevented fusion of the virus and endosomal membranes, inhibiting virus infection. We performed docking experiments, screening a database of natural compounds to identify those likely to bind at this site. Using both inhibition assays of HIV-1-derived pseudovirus cell entry and structural analyses of the complexes of the compounds with GP, we show here that two of these compounds attach in the common binding cavity, out of eight tested. In both cases, two molecules bind in the cavity. The two compounds are chemically similar, but the tighter binder has an additional chlorine atom that forms good halogen bonds to the protein and achieves an IC 50 of 50 nM, making it the most potent GP-binding EBOV inhibitor yet identified, validating our screening approach for the discovery of novel antiviral compounds.
Organizational Affiliation:
Department of Computer and Information Science, Faculty of Science and Technology , University of Macau , E11, Macau 999078 , China.