Nitrocefin acylation of both catalytic serines of penicillin-binding protein 3 from P. aeruginosa
Bellini, D., Dowson, C.G.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peptidoglycan D,D-transpeptidase FtsI | 535 | Pseudomonas aeruginosa PAO1 | Mutation(s): 1 Gene Names: ftsI, pbpB, PA4418 EC: 3.4.16.4 | 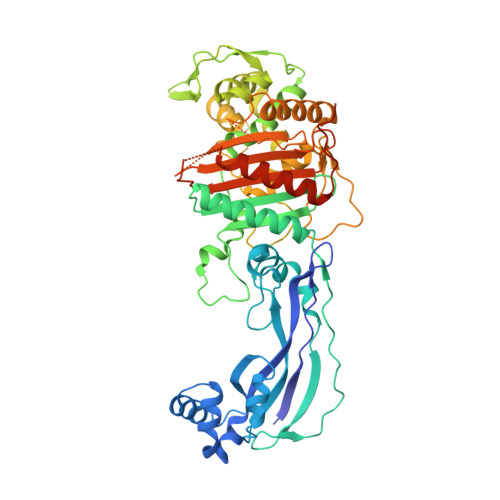 | |
UniProt | |||||
Find proteins for G3XD46 (Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1)) Explore G3XD46 Go to UniProtKB: G3XD46 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G3XD46 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NEF (Subject of Investigation/LOI) Query on NEF | B [auth A], C [auth A] | Nitrocefin - open form C21 H18 N4 O9 S2 PYBZXZZHQKFSGC-CZHQAMEJSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 68.042 | α = 90 |
| b = 83.261 | β = 90 |
| c = 88.733 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DIALS | data reduction |
| Aimless | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Medical Research Council (MRC, United Kingdom) | United Kingdom | grant.MR/P007503/1 |