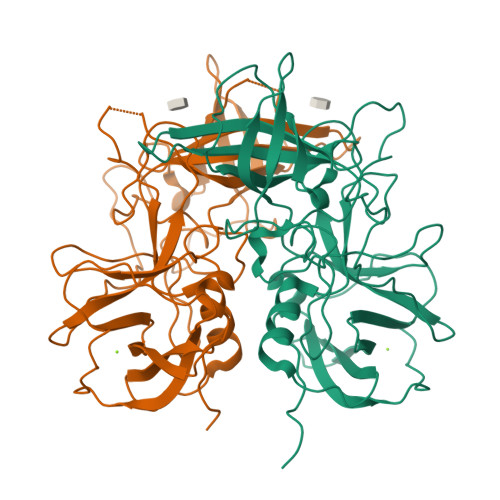
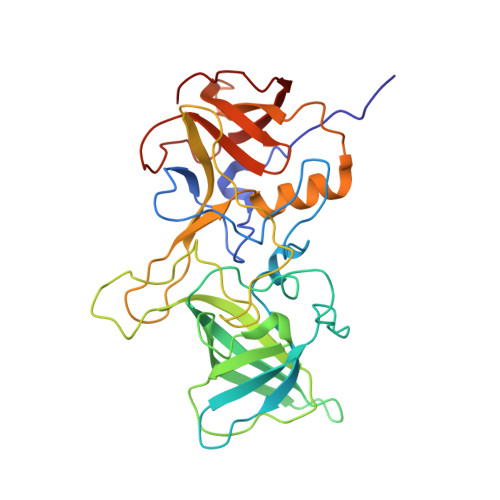
A post-translational modification of human Norovirus capsid protein attenuates glycan binding.
Mallagaray, A., Creutznacher, R., Dulfer, J., Mayer, P.H.O., Grimm, L.L., Orduna, J.M., Trabjerg, E., Stehle, T., Rand, K.D., Blaum, B.S., Uetrecht, C., Peters, T.(2019) Nat Commun 10: 1320-1320
- PubMed: 30899001
- DOI: https://doi.org/10.1038/s41467-019-09251-5
- Primary Citation of Related Structures:
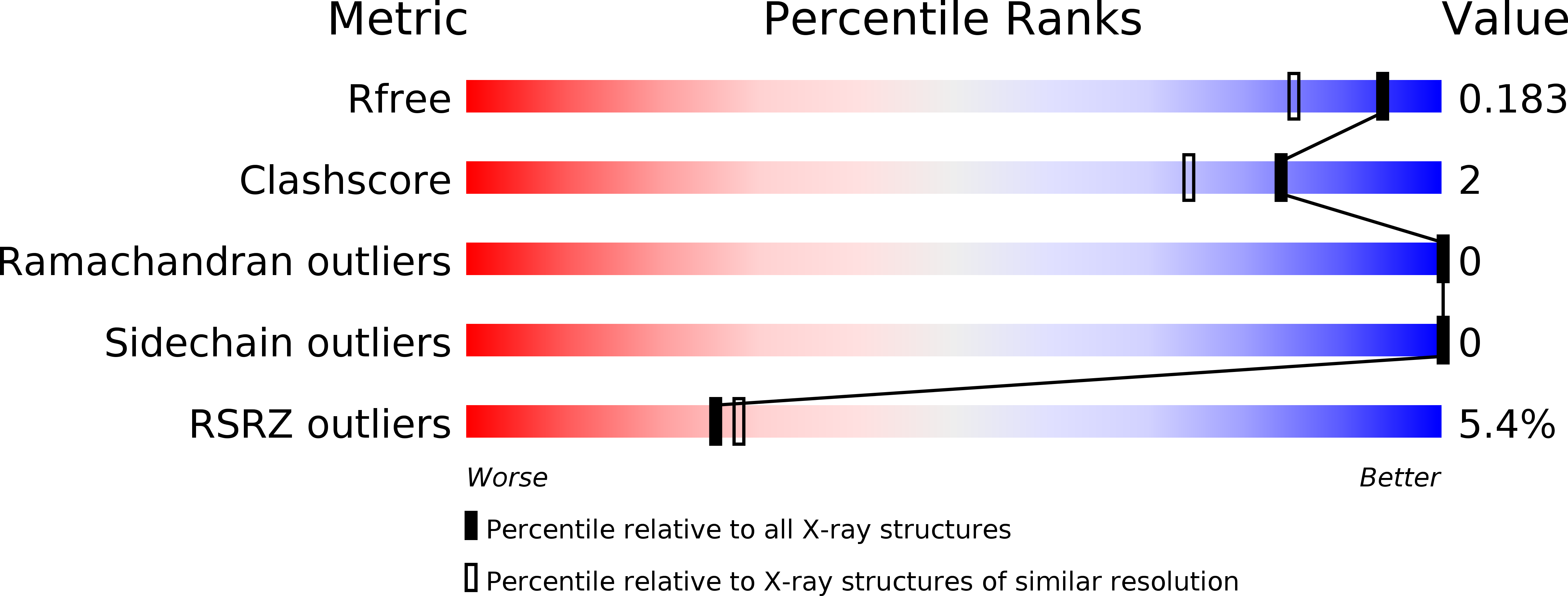
6H9V - PubMed Abstract:
Attachment of human noroviruses to histo blood group antigens (HBGAs) is essential for infection, but how this binding event promotes the infection of host cells is unknown. Here, we employ protein NMR experiments supported by mass spectrometry and crystallography to study HBGA binding to the P-domain of a prevalent virus strain (GII.4). We report a highly selective transformation of asparagine 373, located in an antigenic loop adjoining the HBGA binding site, into an iso-aspartate residue. This spontaneous post-translational modification (PTM) proceeds with an estimated half-life of a few days at physiological temperatures, independent of the presence of HBGAs but dramatically affecting HBGA recognition. Sequence conservation and the surface-exposed position of this PTM suggest an important role in infection and immune recognition for many norovirus strains.
Organizational Affiliation:
Institute of Chemistry and Metabolomics, Center of Structural and Cell Biology in Medicine (CSCM), University of Lübeck, Ratzeburger Allee 160, 23562, Lübeck, Germany.