A Triazolotriazine-Based Dual GSK-3 beta /CK-1 delta Ligand as a Potential Neuroprotective Agent Presenting Two Different Mechanisms of Enzymatic Inhibition.
Redenti, S., Marcovich, I., De Vita, T., Perez, C., De Zorzi, R., Demitri, N., Perez, D.I., Bottegoni, G., Bisignano, P., Bissaro, M., Moro, S., Martinez, A., Storici, P., Spalluto, G., Cavalli, A., Federico, S.(2019) ChemMedChem 14: 310-314
- PubMed: 30548443
- DOI: https://doi.org/10.1002/cmdc.201800778
- Primary Citation of Related Structures:
6H0U - PubMed Abstract:
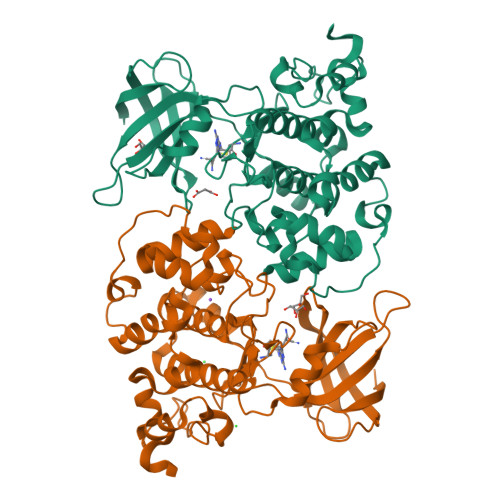
Glycogen synthase kinase 3β (GSK-3β) and casein kinase 1δ (CK-1δ) are emerging targets for the treatment of neuroinflammatory disorders, including Parkinson's disease. An inhibitor able to target these two kinases was developed by docking-based design. Compound 12, 3-(7-amino-5-(cyclohexylamino)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-2-yl)-2-cyanoacrylamide, showed combined inhibitory activity against GSK-3β and CK-1δ [IC 50 (GSK-3β)=0.17 μm; IC 50 (CK-1δ)=0.68 μm]. In particular, classical ATP competition was observed against CK-1δ, and a co-crystal of compound 12 inside GSK-3β confirmed a covalent interaction between the cyanoacrylamide warhead and Cys199, which could help in the development of more potent covalent inhibitors of GSK-3β. Preliminary studies on in vitro models of Parkinson's disease revealed that compound 12 is not cytotoxic and shows neuroprotective activity. These results encourage further investigations to validate GSK-3β/CK-1δ inhibition as a possible new strategy to treat neuroinflammatory/degenerative diseases.
Organizational Affiliation:
Department of Chemical and Pharmaceutical Sciences, University of Trieste, Via Licio Giorgeri 1, 34127, Trieste, Italy.