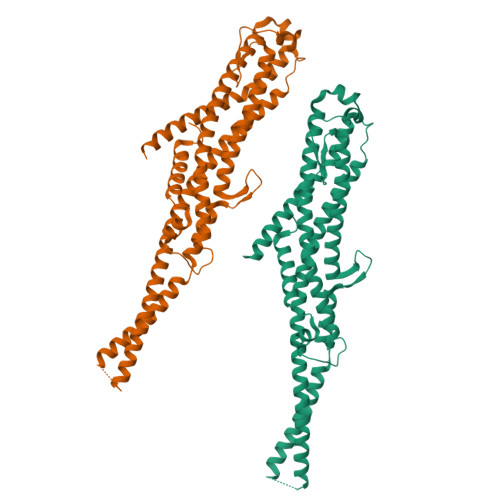
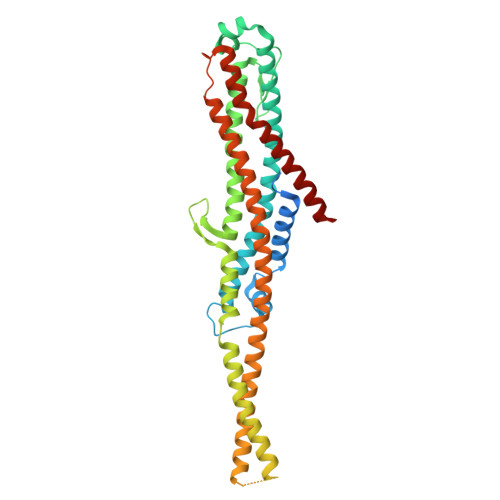
Membrane insertion of alpha-xenorhabdolysin in near-atomic detail.
Schubert, E., Vetter, I.R., Prumbaum, D., Penczek, P.A., Raunser, S.(2018) Elife 7
- PubMed: 30010541
- DOI: https://doi.org/10.7554/eLife.38017
- Primary Citation of Related Structures:
6GY6, 6GY7, 6GY8 - PubMed Abstract:
α-Xenorhabdolysins (Xax) are α-pore-forming toxins (α-PFT) that form 1-1.3 MDa large pore complexes to perforate the host cell membrane. PFTs are used by a variety of bacterial pathogens to attack host cells. Due to the lack of structural information, the molecular mechanism of action of Xax toxins is poorly understood. Here, we report the cryo-EM structure of the XaxAB pore complex from Xenorhabdus nematophila and the crystal structures of the soluble monomers of XaxA and XaxB. The structures reveal that XaxA and XaxB are built similarly and appear as heterodimers in the 12-15 subunits containing pore, classifying XaxAB as bi-component α-PFT. Major conformational changes in XaxB, including the swinging out of an amphipathic helix are responsible for membrane insertion. XaxA acts as an activator and stabilizer for XaxB that forms the actual transmembrane pore. Based on our results, we propose a novel structural model for the mechanism of Xax intoxication.
Organizational Affiliation:
Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, Dortmund, Germany.