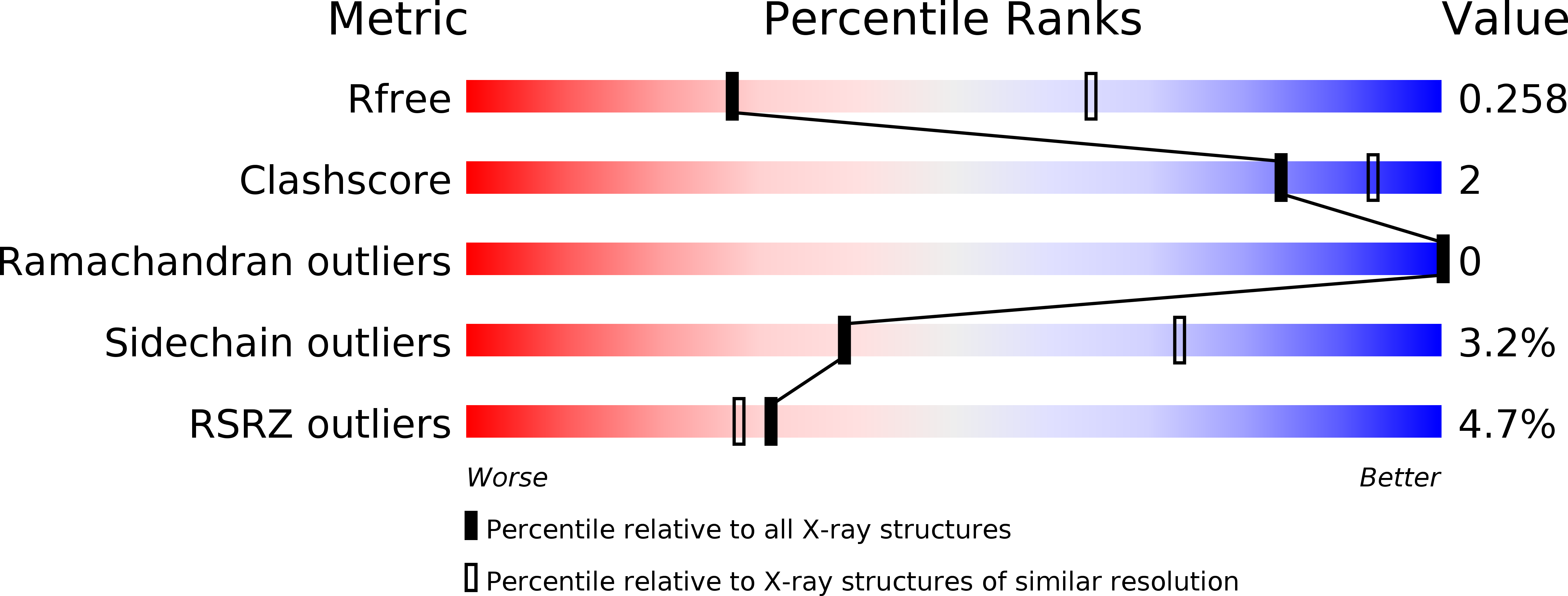
Development of alkyl glycerone phosphate synthase inhibitors: Structure-activity relationship and effects on ether lipids and epithelial-mesenchymal transition in cancer cells.
Stazi, G., Battistelli, C., Piano, V., Mazzone, R., Marrocco, B., Marchese, S., Louie, S.M., Zwergel, C., Antonini, L., Patsilinakos, A., Ragno, R., Viviano, M., Sbardella, G., Ciogli, A., Fabrizi, G., Cirilli, R., Strippoli, R., Marchetti, A., Tripodi, M., Nomura, D.K., Mattevi, A., Mai, A., Valente, S.(2018) Eur J Med Chem 163: 722-735
- PubMed: 30576903
- DOI: https://doi.org/10.1016/j.ejmech.2018.11.050
- Primary Citation of Related Structures:
6GOU - PubMed Abstract:
In aggressive tumors, alkylglyceronephosphate synthase (AGPS) controls cellular ether phospholipid utilization and metabolism to promote cancer cell proliferation and motility. SAR studies on the first-in-class AGPS inhibitor 1, discovered by our group, led to the 2,6-difluoro analog 2i which showed higher binding affinity than 1in vitro. In 231MFP cancer cells, 2i reduced ether lipids levels and cell migration rate. When tested in PC-3 and MDA-MB-231 cancer cells, 2i specifically impaired epithelial to mesenchymal transition (EMT) by modulating E-cadherin, Snail and MMP2 expression levels. Moreover, the combination of siRNAs against AGPS and 2i provided no additive effect, confirming that the modulation of 2i on EMT specifically relies on AGPS inhibition. Finally, this compound also affected cancer cell proliferation especially in MDA-MB-231 cells expressing higher AGPS level, whereas it provided negligible effects on MeT5A, a non-tumorigenic cell line, thus showing cancer specificity.
Organizational Affiliation:
Department of Chemistry and Technologies of Drugs, Sapienza University of Rome, P. le A. Moro 5, 00185, Rome, Italy.