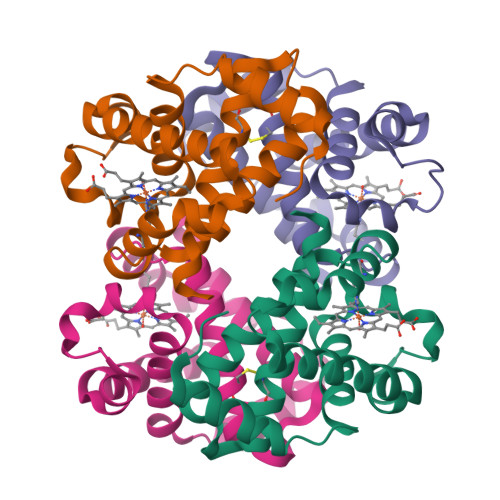
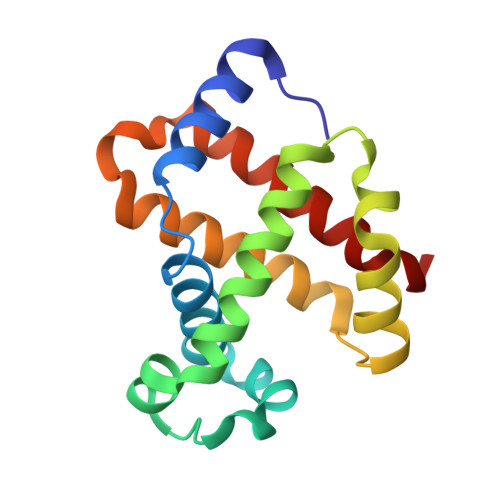
Crystal structure of the ferric homotetrameric beta4human hemoglobin.
Mazzarella, L., Merlino, A., Balasco, N., Balsamo, A., Vergara, A.(2018) Biophys Chem 240: 9-14
- PubMed: 29857171
- DOI: https://doi.org/10.1016/j.bpc.2018.05.003
- Primary Citation of Related Structures:
6FQF - PubMed Abstract:
Spectroscopic studies carried out in the early seventies have shown that the β-homotetramer of human hemoglobin (β 4 -HbA) in the ferric state is a mixture of aquomet and bis-histidyl forms. Here we present the first crystal structure, solved at 2.10 Å resolution, of the oxidized form of β 4 -HbA. The overall quaternary structure of the protein in the ferric state is virtually indistinguishable from that of the ferrous deoxygenated and carbomonoxy forms. The structure reveals that the four hemes are exclusively in an aquomet coordination, without any trace of bis-histidyl coordination. The oxidation of β 4 -HbA is associated with the formation of a disulfide bridge between residues Cys112(G14) of β 1 /β 4 and β 2 /β 3 chains. The coordination state of β 4 -HbA has been compared to that known for other organisms that exhibit bis-histidyl heme coordination in the β 4 state. This occurrence has been discussed in terms of different organism physiology.
Organizational Affiliation:
Department of Chemical Sciences, University of Naples "Federico II", Naples, Italy; Accademia di Scienze Fisiche e Matematiche della Società Nazionale di Scienze, Lettere ed Arti in Napoli, Naples, Italy.