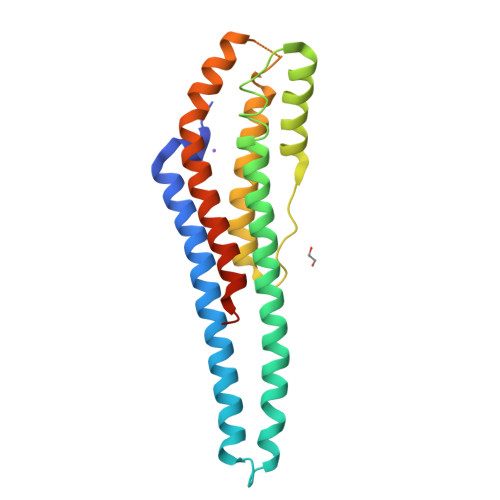
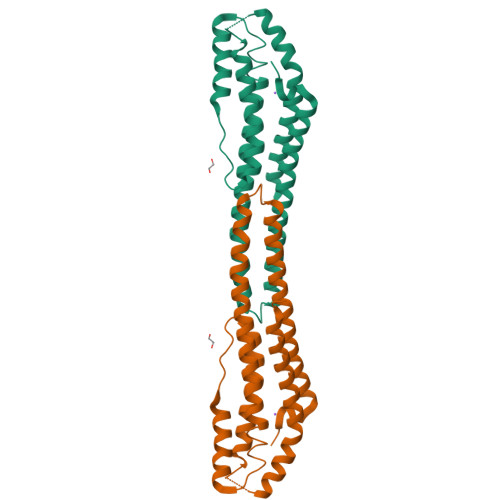
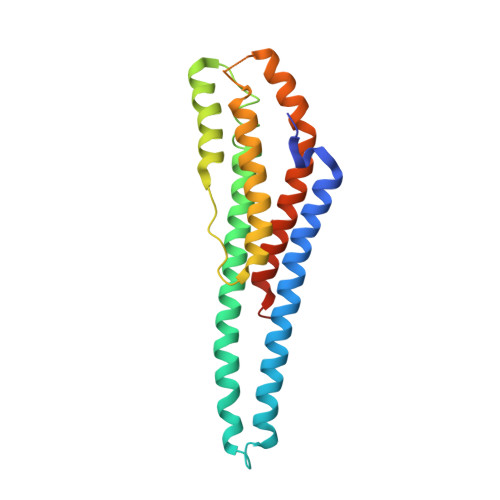
Structure and properties of AB21, a novel Agaricus bisporus protein with structural relation to bacterial pore-forming toxins.
Komarek, J., Ivanov Kavkova, E., Houser, J., Horackova, A., Zdanska, J., Demo, G., Wimmerova, M.(2018) Proteins 86: 897-911
- PubMed: 29722060
- DOI: https://doi.org/10.1002/prot.25522
- Primary Citation of Related Structures:
6FPD - PubMed Abstract:
We report the characterization of the dimeric protein AB21 from Agaricus bisporus, one of the most commonly and widely consumed mushrooms in the world. The protein shares no significant sequence similarity with any protein of known function, and it is the first characterized member of its protein family. The coding sequence of the ab21 gene was determined and the protein was expressed in E. coli in a recombinant form. We demonstrated a high thermal and pH stability of AB21 and proved the weak affinity of the protein to divalent ions of some transition metals (nickel, zinc, cadmium, and cobalt). The reported crystallographic structure exhibits an interesting rod-like helical bundle fold with structural similarity to bacterial toxins of the ClyA superfamily. By immunostaining, we demonstrated an abundance of AB21 in the fruiting bodies of A. bisporus.
Organizational Affiliation:
Central European Institute of Technology, Masaryk University, Kamenice 5, Brno, 62500, Czech Republic.