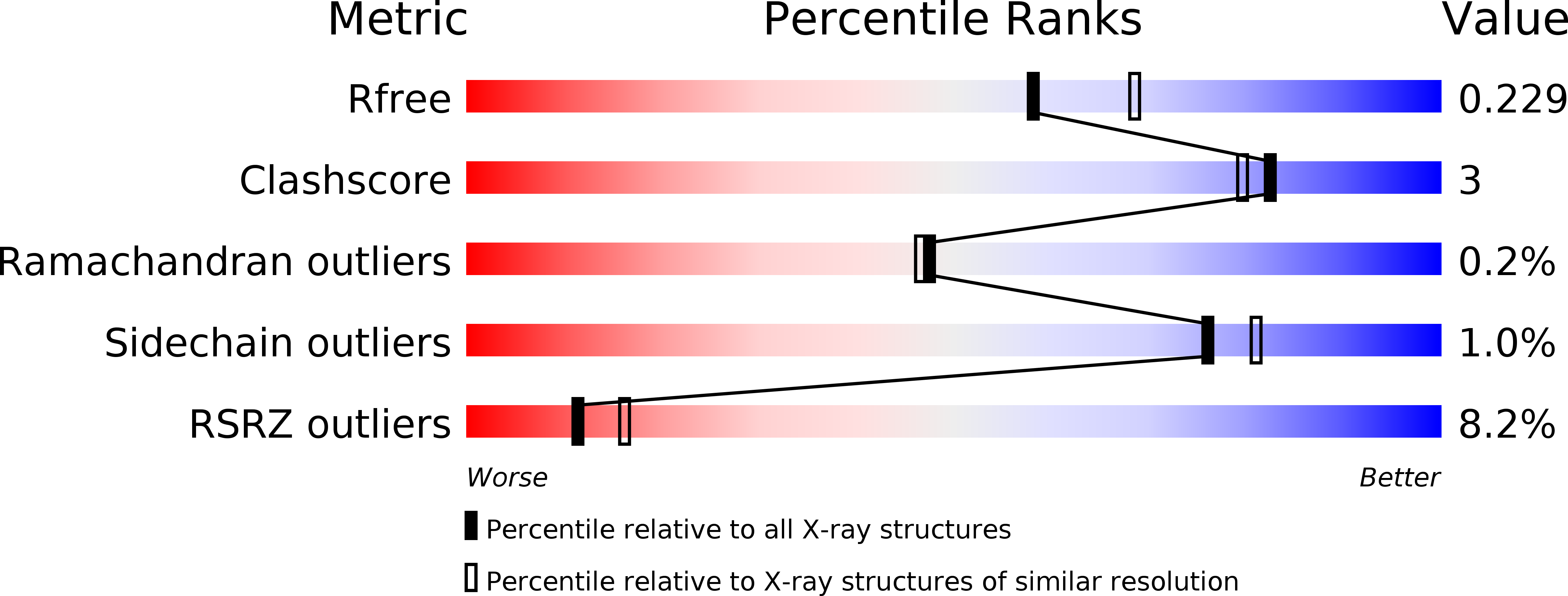
A brain-permeable inhibitor of the neurodegenerative disease target kynurenine 3-monooxygenase prevents accumulation of neurotoxic metabolites.
Zhang, S., Sakuma, M., Deora, G.S., Levy, C.W., Klausing, A., Breda, C., Read, K.D., Edlin, C.D., Ross, B.P., Wright Muelas, M., Day, P.J., O'Hagan, S., Kell, D.B., Schwarcz, R., Leys, D., Heyes, D.J., Giorgini, F., Scrutton, N.S.(2019) Commun Biol 2: 271-271
- PubMed: 31372510
- DOI: https://doi.org/10.1038/s42003-019-0520-5
- Primary Citation of Related Structures:
6FOX, 6FOY, 6FOZ, 6FP0, 6FP1, 6FPH - PubMed Abstract:
Dysregulation of the kynurenine pathway (KP) leads to imbalances in neuroactive metabolites associated with the pathogenesis of several neurodegenerative disorders, including Huntington's disease (HD). Inhibition of the enzyme kynurenine 3-monooxygenase (KMO) in the KP normalises these metabolic imbalances and ameliorates neurodegeneration and related phenotypes in several neurodegenerative disease models. KMO is thus a promising candidate drug target for these disorders, but known inhibitors are not brain permeable. Here, 19 new KMO inhibitors have been identified. One of these ( 1 ) is neuroprotective in a Drosophila HD model but is minimally brain penetrant in mice. The prodrug variant ( 1b ) crosses the blood-brain barrier, releases 1 in the brain, thereby lowering levels of 3-hydroxykynurenine, a toxic KP metabolite linked to neurodegeneration. Prodrug 1b will advance development of targeted therapies against multiple neurodegenerative and neuroinflammatory diseases in which KP likely plays a role, including HD, Alzheimer's disease, and Parkinson's disease.
Organizational Affiliation:
1Manchester Institute of Biotechnology and School of Chemistry, The University of Manchester, Manchester, M1 7DN UK.