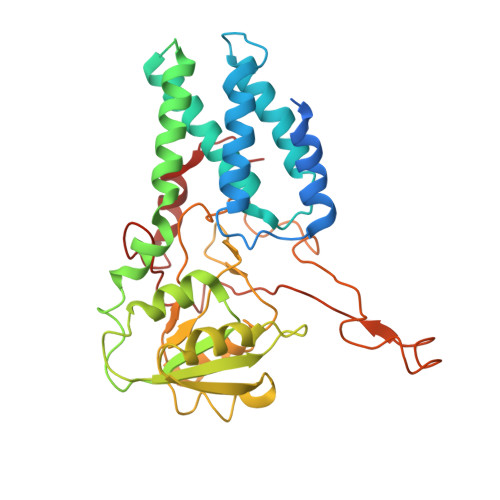
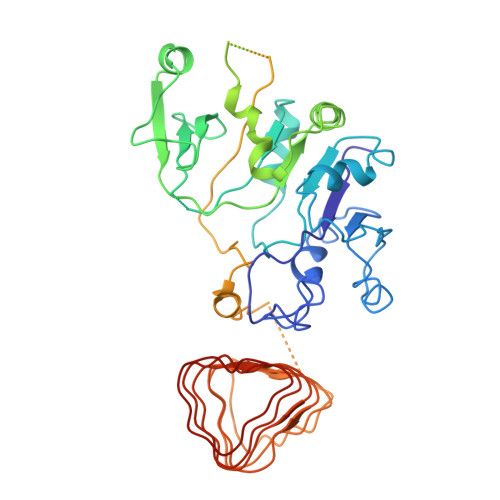
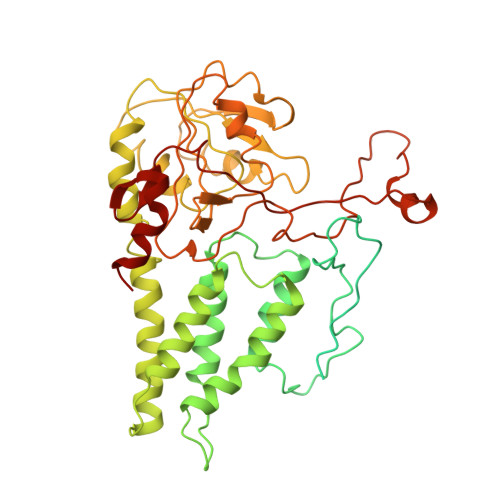
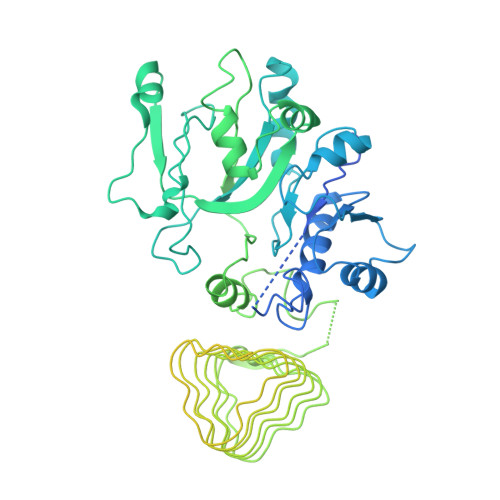
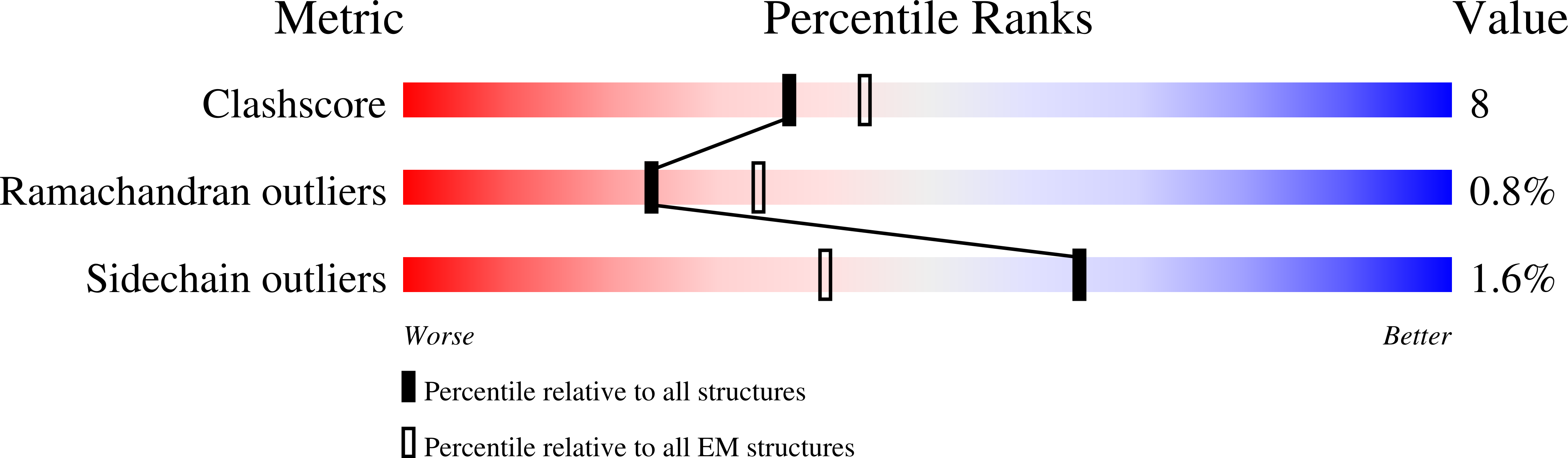Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B.
Zyryanova, A.F., Weis, F., Faille, A., Alard, A.A., Crespillo-Casado, A., Sekine, Y., Harding, H.P., Allen, F., Parts, L., Fromont, C., Fischer, P.M., Warren, A.J., Ron, D.(2018) Science 359: 1533-1536
- PubMed: 29599245
- DOI: https://doi.org/10.1126/science.aar5129
- Primary Citation of Related Structures:
6EZO - PubMed Abstract:
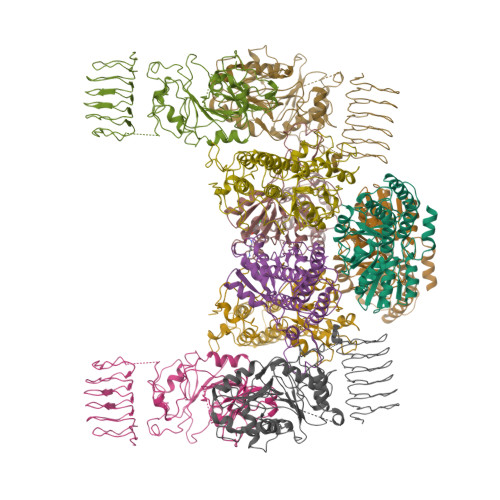
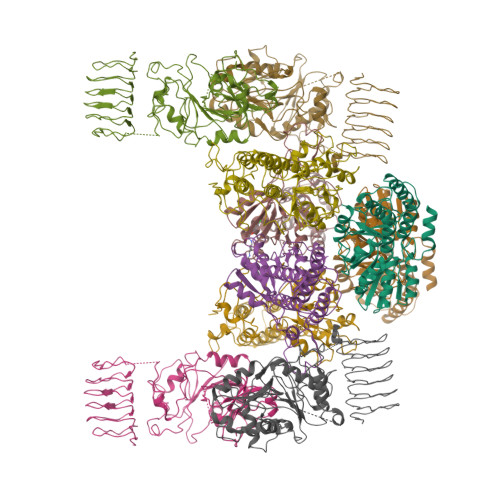
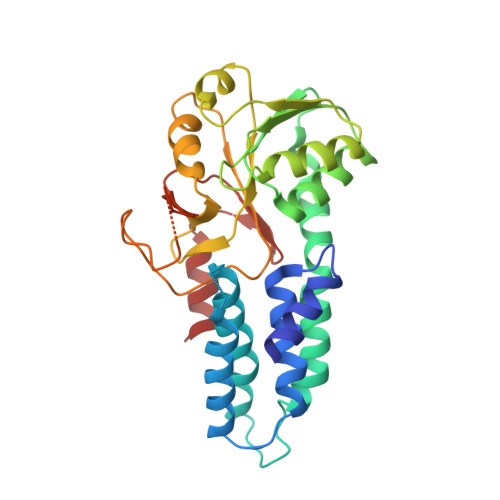
The integrated stress response (ISR) is a conserved translational and transcriptional program affecting metabolism, memory, and immunity. The ISR is mediated by stress-induced phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) that attenuates the guanine nucleotide exchange factor eIF2B. A chemical inhibitor of the ISR, ISRIB, reverses the attenuation of eIF2B by phosphorylated eIF2α, protecting mice from neurodegeneration and traumatic brain injury. We describe a 4.1-angstrom-resolution cryo-electron microscopy structure of human eIF2B with an ISRIB molecule bound at the interface between the β and δ regulatory subunits. Mutagenesis of residues lining this pocket altered the hierarchical cellular response to ISRIB analogs in vivo and ISRIB binding in vitro. Our findings point to a site in eIF2B that can be exploited by ISRIB to regulate translation.
Organizational Affiliation:
Cambridge Institute for Medical Research, University of Cambridge, Cambridge CB2 0XY, UK. az310@cam.ac.uk ajw1000@cam.ac.uk dr360@medschl.cam.ac.uk.