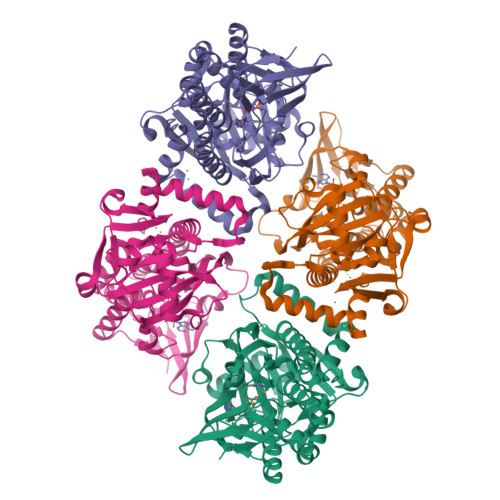
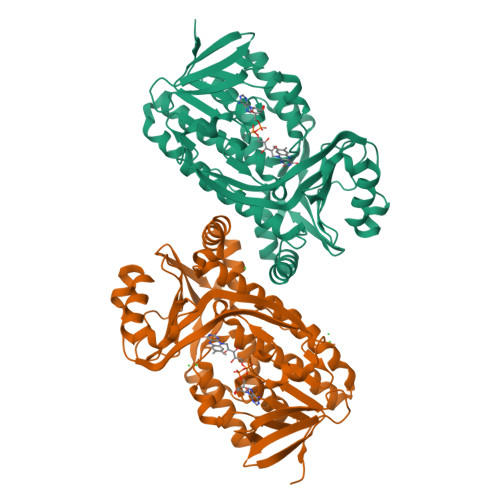
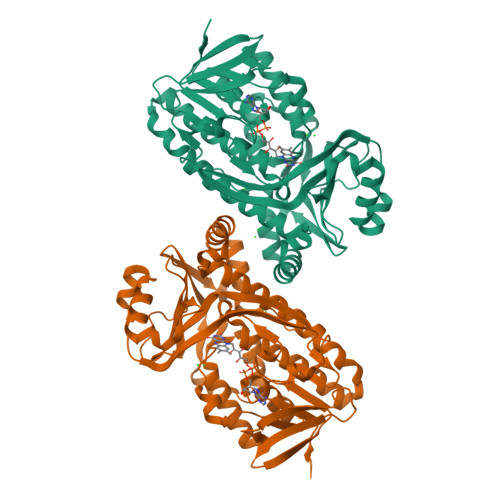
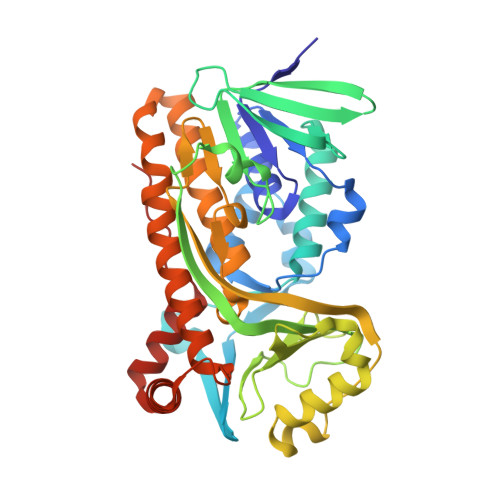
Structural comparison of p-hydroxybenzoate hydroxylase (PobA) from Pseudomonas putida with PobA from other Pseudomonas spp. and other monooxygenases.
Lazar, J.T., Shuvalova, L., Rosas-Lemus, M., Kiryukhina, O., Satchell, K.J.F., Minasov, G.(2019) Acta Crystallogr F Struct Biol Commun 75: 507-514
- PubMed: 31282871
- DOI: https://doi.org/10.1107/S2053230X19008653
- Primary Citation of Related Structures:
6DLL - PubMed Abstract:
The crystal structure is reported of p-hydroxybenzoate hydroxylase (PobA) from Pseudomonas putida, a possible drug target to combat tetracycline resistance, in complex with flavin adenine dinucleotide (FAD). The structure was refined at 2.2 Å resolution with four polypeptide chains in the asymmetric unit. Based on the results of pairwise structure alignments, PobA from P. putida is structurally very similar to PobA from P. fluorescens and from P. aeruginosa. Key residues in the FAD-binding and substrate-binding sites of PobA are highly conserved spatially across the proteins from all three species. Additionally, the structure was compared with two enzymes from the broader class of oxygenases: 2-hydroxybiphenyl 3-monooxygenase (HbpA) from P. nitroreducens and 2-methyl-3-hydroxypyridine-5-carboxylic acid oxygenase (MHPCO) from Mesorhizobium japonicum. Despite having only 14% similarity in their primary sequences, pairwise structure alignments of PobA from P. putida with HbpA from P. nitroreducens and MHPCO from M. japonicum revealed local similarities between these structures. Key secondary-structure elements important for catalysis, such as the βαβ fold, β-sheet wall and α12 helix, are conserved across this expanded class of oxygenases.
Organizational Affiliation:
Department of Chemical and Biological Engineering, Northwestern University, Evanston, IL 60201, USA.