Structure of Ddi2, a highly inducible detoxifying metalloenzyme fromSaccharomyces cerevisiae.
Li, J., Jia, Y., Lin, A., Hanna, M., Chelico, L., Xiao, W., Moore, S.A.(2019) J Biological Chem 294: 10674-10685
- PubMed: 31152065
- DOI: https://doi.org/10.1074/jbc.RA118.006394
- Primary Citation of Related Structures:
6DK9, 6DKA, 6DKC, 6DKD - PubMed Abstract:
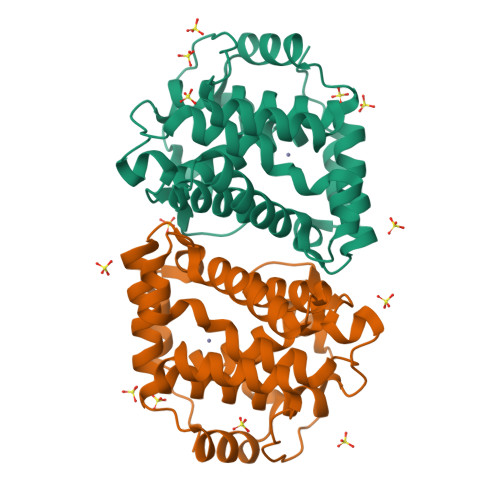
Cyanamide (H 2 N-CN) is used to break bud dormancy in woody plants and to deter alcohol use in humans. The biological effects of cyanamide in both these cases require the enzyme catalase. We previously demonstrated that Saccharomyces cerevisiae exposed to cyanamide resulted in strong induction of DDI2 gene expression. Ddi2 enzymatically hydrates cyanamide to urea and belongs to the family of HD-domain metalloenzymes (named after conserved active-site metal-binding His and Asp residues). Here, we report the X-ray structure of yeast Ddi2 to 2.6 Å resolution, revealing that Ddi2 is a dimeric zinc metalloenzyme. We also confirm that Ddi2 shares structural similarity with other known HD-domain proteins. HD residues His-55, His-88, and Asp-89 coordinate the active-site zinc, and the fourth zinc ligand is a water/hydroxide molecule. Other HD domain enzymes have a second aspartate metal ligand, but in Ddi2 this residue (Thr-157) does not interact with the zinc ion. Several Ddi2 active-site point mutations exhibited reduced catalytic activity. We kinetically and structurally characterized H137N and T157V mutants of Ddi2. A cyanamide soak of the Ddi2-T157V enzyme revealed cyanamide bound directly to the Zn 2+ ion, having displaced the zinc-bound water molecule. The mode of cyanamide binding to Ddi2 resembles cyanamide binding to the active-site zinc of carbonic anhydrase, a known cyanamide hydratase. Finally, we observed that the sensitivity of ddi2 Δ ddi3 Δ to cyanamide was not rescued by plasmids harboring ddi2-H137N or ddi2-TI57V variants, demonstrating that yeast cells require a functioning cyanamide hydratase to overcome cyanamide-induced growth defects.
Organizational Affiliation:
From the Department of Biochemistry, Microbiology and Immunology, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5E5, Canada and.