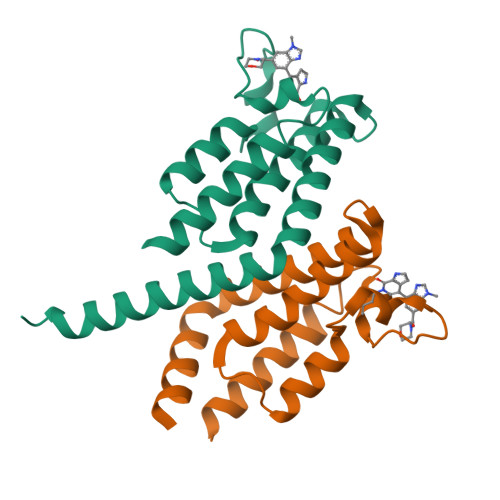
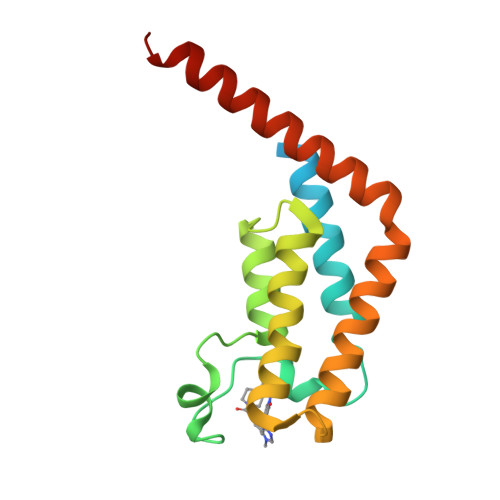
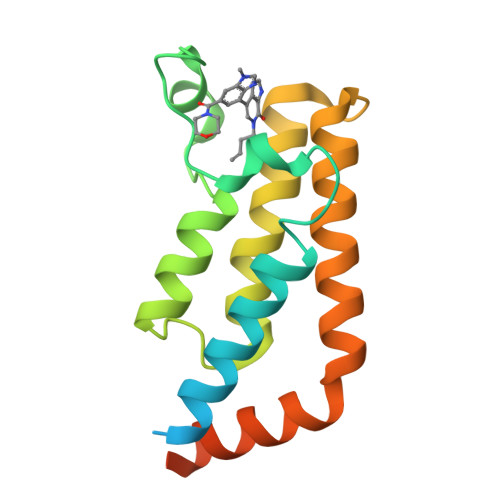
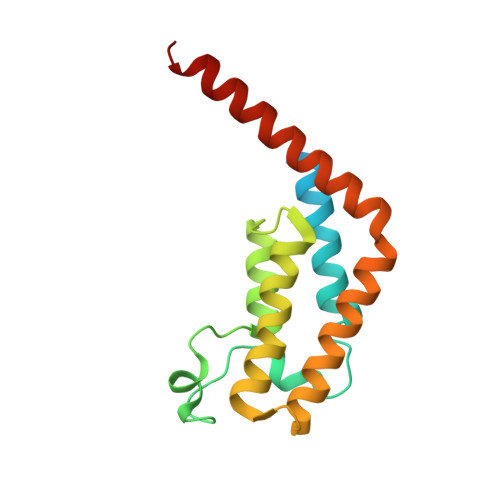
GNE-371, a Potent and Selective Chemical Probe for the Second Bromodomains of Human Transcription-Initiation-Factor TFIID Subunit 1 and Transcription-Initiation-Factor TFIID Subunit 1-like.
Wang, S., Tsui, V., Crawford, T.D., Audia, J.E., Burdick, D.J., Beresini, M.H., Cote, A., Cummings, R., Duplessis, M., Flynn, E.M., Hewitt, M.C., Huang, H.R., Jayaram, H., Jiang, Y., Joshi, S., Murray, J., Nasveschuk, C.G., Pardo, E., Poy, F., Romero, F.A., Tang, Y., Taylor, A.M., Wang, J., Xu, Z., Zawadzke, L.E., Zhu, X., Albrecht, B.K., Magnuson, S.R., Bellon, S., Cochran, A.G.(2018) J Med Chem 61: 9301-9315
- PubMed: 30289257
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01225
- Primary Citation of Related Structures:
6DF4, 6DF7 - PubMed Abstract:
The biological functions of the dual bromodomains of human transcription-initiation-factor TFIID subunit 1 (TAF1(1,2)) remain unknown, although TAF1 has been identified as a potential target for oncology research. Here, we describe the discovery of a potent and selective in vitro tool compound for TAF1(2), starting from a previously reported lead. A cocrystal structure of lead compound 2 bound to TAF1(2) enabled structure-based design and structure-activity-relationship studies that ultimately led to our in vitro tool compound, 27 (GNE-371). Compound 27 binds TAF1(2) with an IC 50 of 10 nM while maintaining excellent selectivity over other bromodomain-family members. Compound 27 is also active in a cellular-TAF1(2) target-engagement assay (IC 50 = 38 nM) and exhibits antiproliferative synergy with the BET inhibitor JQ1, suggesting engagement of endogenous TAF1 by 27 and further supporting the use of 27 in mechanistic and target-validation studies.
Organizational Affiliation:
Genentech Inc. , 1 DNA Way , South San Francisco , California 94080 , United States.