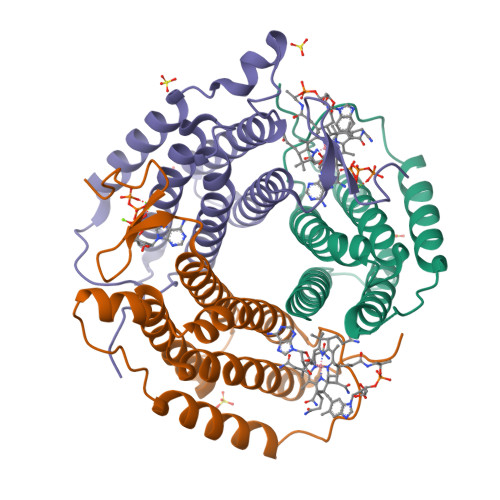
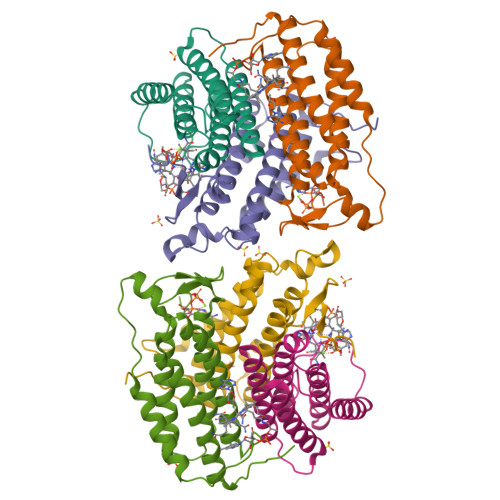
Sacrificial Cobalt-Carbon Bond Homolysis in Coenzyme B12as a Cofactor Conservation Strategy.
Campanello, G.C., Ruetz, M., Dodge, G.J., Gouda, H., Gupta, A., Twahir, U.T., Killian, M.M., Watkins, D., Rosenblatt, D.S., Brunold, T.C., Warncke, K., Smith, J.L., Banerjee, R.(2018) J Am Chem Soc 140: 13205-13208
- PubMed: 30282455
- DOI: https://doi.org/10.1021/jacs.8b08659
- Primary Citation of Related Structures:
6D5K, 6D5X - PubMed Abstract:
A sophisticated intracellular trafficking pathway in humans is used to tailor vitamin B 12 into its active cofactor forms, and to deliver it to two known B 12 -dependent enzymes. Herein, we report an unexpected strategy for cellular retention of B 12 , an essential and reactive cofactor. If methylmalonyl-CoA mutase is unavailable to accept the coenzyme B 12 product of adenosyltransferase, the latter catalyzes homolytic scission of the cobalt-carbon bond in an unconventional reversal of the nucleophilic displacement reaction that was used to make it. The resulting homolysis product binds more tightly to adenosyltransferase than does coenzyme B 12 , facilitating cofactor retention. We have trapped, and characterized spectroscopically, an intermediate in which the cobalt-carbon bond is weakened prior to being broken. The physiological relevance of this sacrificial catalytic activity for cofactor retention is supported by the significantly lower coenzyme B 12 concentration in patients with dysfunctional methylmalonyl-CoA mutase but normal adenosyltransferase activity.
Organizational Affiliation:
Department of Biological Chemistry , University of Michigan , Ann Arbor , Michigan 48109-0600 , United States.