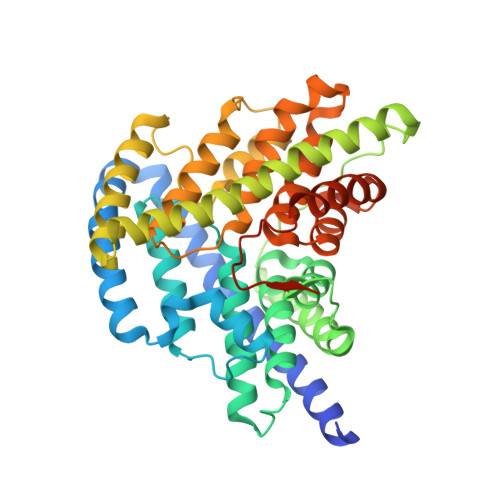
A CLC-type F-/H+antiporter in ion-swapped conformations.
Last, N.B., Stockbridge, R.B., Wilson, A.E., Shane, T., Kolmakova-Partensky, L., Koide, A., Koide, S., Miller, C.(2018) Nat Struct Mol Biol 25: 601-606
- PubMed: 29941917
- DOI: https://doi.org/10.1038/s41594-018-0082-0
- Primary Citation of Related Structures:
6D0J, 6D0K, 6D0N - PubMed Abstract:
Fluoride/proton antiporters of the CLC F family combat F - toxicity in bacteria by exporting this halide from the cytoplasm. These transporters belong to the widespread CLC superfamily but display transport properties different from those of the well-studied Cl - /H + antiporters. Here, we report a structural and functional investigation of these F - -transport proteins. Crystal structures of a CLC F homolog from Enterococcus casseliflavus are captured in two conformations with simultaneous accessibility of F - and H + ions via separate pathways on opposite sides of the membrane. Manipulation of a key glutamate residue critical for H + and F - transport reverses the anion selectivity of transport; replacement of the glutamate with glutamine or alanine completely inhibits F - and H + transport while allowing for rapid uncoupled flux of Cl - . The structural and functional results lead to a 'windmill' model of CLC antiport wherein F - and H + simultaneously move through separate ion-specific pathways that switch sidedness during the transport cycle.
Organizational Affiliation:
Department of Biochemistry, Howard Hughes Medical Institute, Brandeis University, Waltham, MA, USA.