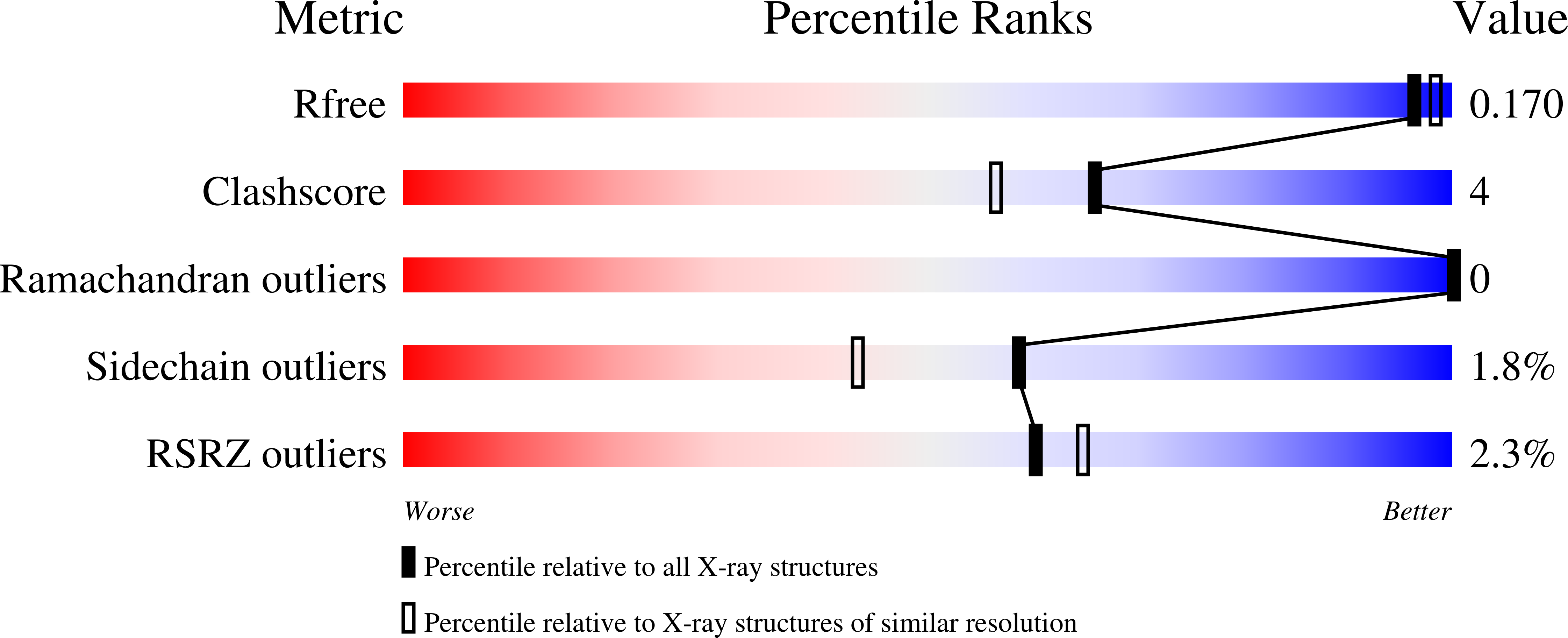
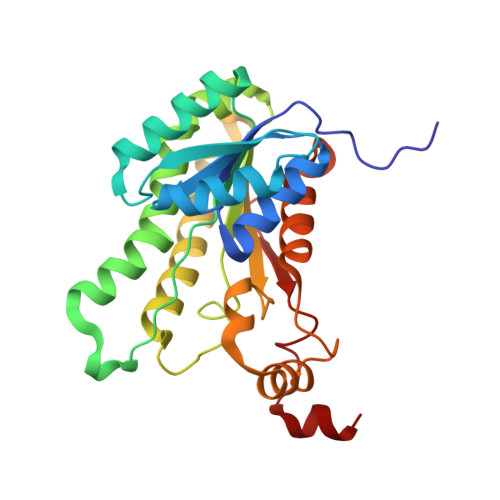
Structure and Kinetics of the S-(+)-1-Amino-2-propanol Dehydrogenase from the RMM Microcompartment of Mycobacterium smegmatis.
Mallette, E., Kimber, M.S.(2018) Biochemistry 57: 3780-3789
- PubMed: 29757625
- DOI: https://doi.org/10.1021/acs.biochem.8b00464
- Primary Citation of Related Structures:
6CI8, 6CI9 - PubMed Abstract:
S-(+)-1-Amino-2-propanol dehydrogenase (APDH) is a short-chain dehydrogenase/reductase associated with the incompletely characterized Rhodococcus and Mycobacterium bacterial microcompartment (RMM). We enzymatically characterized the APDH from M. smegmatis and showed it is highly selective, with a low micromolar K m for S-(+)-1-amino-2-propanol and specificity for NADP(H). A paralogous enzyme from a nonmicrocompartment-associated operon in the same organism was also shown to have a similar activity. We determined the structure of APDH in both apo form (at 1.7 Å) and as a ternary enzyme complex with NADP + and aminoacetone (at 1.9 Å). Recognition of aminoacetone was mediated by strong hydrogen bonds to the amino group by Thr145 and by Glu251 from the C-terminus of an adjacent protomer. The substrate binding site entirely encloses the substrate, with close contacts between the aminoacetone methyl group and Phe95, Trp154, and Leu195. Kinetic characterization of several of these residues confirm their importance in enzyme functioning. Bioinformatics analysis of APDH homologues implies that many nonmicrocompartment APDH orthologues partake in an aminoacetone degradation pathway that proceeds via an aminopropanol O-phosphate phospholyase. RMM microcompartments may mediate a similar pathway, though possibly with differences in the details of the pathway that necessitates encapsulation behind a shell.
Organizational Affiliation:
Department of Molecular and Cellular Biology , University of Guelph , Guelph , Ontario N1G 2W1 , Canada.