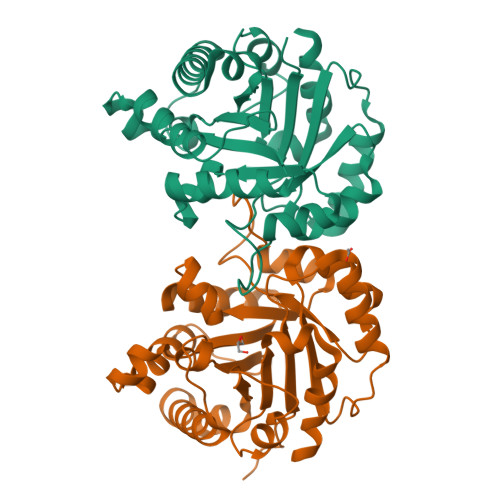
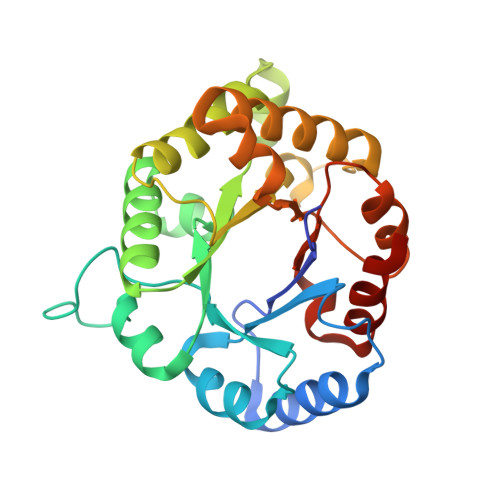
Structure and conformational stability of the triosephosphate isomerase from Zea mays. Comparison with the chemical unfolding pathways of other eukaryotic TIMs.
Romero-Romero, S., Becerril-Sesin, L.A., Costas, M., Rodriguez-Romero, A., Fernandez-Velasco, D.A.(2018) Arch Biochem Biophys 658: 66-76
- PubMed: 30261166
- DOI: https://doi.org/10.1016/j.abb.2018.09.022
- Primary Citation of Related Structures:
6CG9 - PubMed Abstract:
We studied the structure, function and thermodynamic properties for the unfolding of the Triosephosphate isomerase (TIM) from Zea mays (ZmTIM). ZmTIM shows a catalytic efficiency close to the diffusion limit. Native ZmTIM is a dimer that dissociates upon dilution into inactive and unfolded monomers. Its thermal unfolding is irreversible with a T m of 61.6 ± 1.4 °C and an activation energy of 383.4 ± 11.5 kJ mol -1 . The urea-induced unfolding of ZmTIM is reversible. Transitions followed by catalytic activity and spectroscopic properties are monophasic and superimposable, indicating that ZmTIM unfolds/refolds in a two-state behavior with an unfolding ΔG° (H20) = 99.8 ± 5.3 kJ mol -1 . This contrasts with most other studied TIMs, where folding intermediates are common. The three-dimensional structure of ZmTIM was solved at 1.8 Å. A structural comparison with other eukaryotic TIMs shows a similar number of intramolecular and intermolecular interactions. Interestingly the number of interfacial water molecules found in ZmTIM is lower than those observed in most TIMs that show folding intermediates. Although with the available data, there is no clear correlation between structural properties and the number of equilibrium intermediates in the unfolding of TIM, the identification of such structural properties should increase our understanding of folding mechanisms.
Organizational Affiliation:
Laboratorio de Fisicoquímica e Ingeniería de Proteínas, Departamento de Bioquímica, Facultad de Medicina, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510, Mexico.