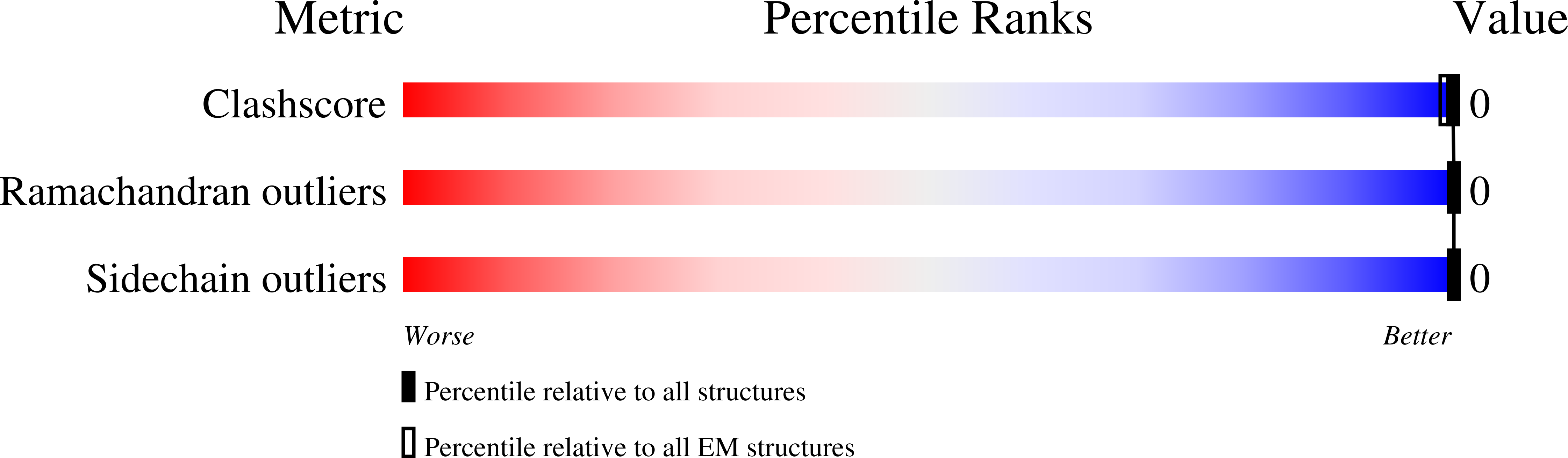Atomic structures of TDP-43 LCD segments and insights into reversible or pathogenic aggregation.
Guenther, E.L., Cao, Q., Trinh, H., Lu, J., Sawaya, M.R., Cascio, D., Boyer, D.R., Rodriguez, J.A., Hughes, M.P., Eisenberg, D.S.(2018) Nat Struct Mol Biol 25: 463-471
- PubMed: 29786080
- DOI: https://doi.org/10.1038/s41594-018-0064-2
- Primary Citation of Related Structures:
5WHN, 5WHP, 5WIA, 5WIQ, 5WKB, 5WKD, 6CB9, 6CEW, 6CF4, 6CFH - PubMed Abstract:
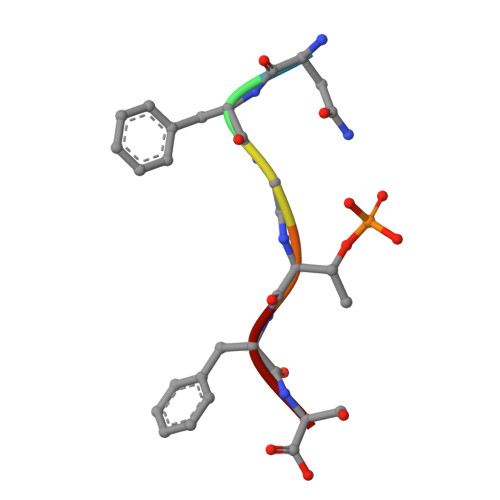
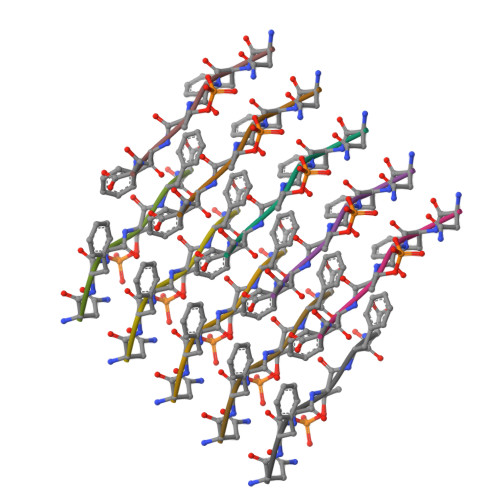
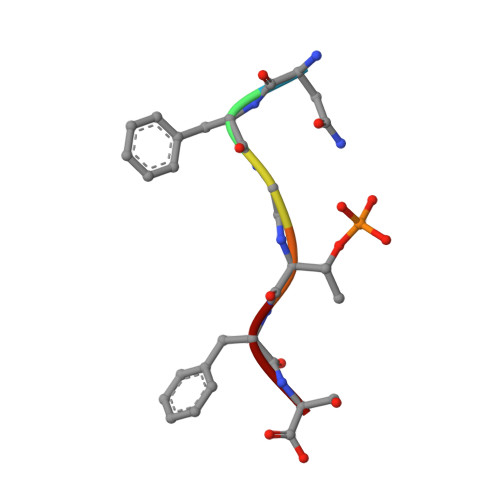
The normally soluble TAR DNA-binding protein 43 (TDP-43) is found aggregated both in reversible stress granules and in irreversible pathogenic amyloid. In TDP-43, the low-complexity domain (LCD) is believed to be involved in both types of aggregation. To uncover the structural origins of these two modes of β-sheet-rich aggregation, we have determined ten structures of segments of the LCD of human TDP-43. Six of these segments form steric zippers characteristic of the spines of pathogenic amyloid fibrils; four others form LARKS, the labile amyloid-like interactions characteristic of protein hydrogels and proteins found in membraneless organelles, including stress granules. Supporting a hypothetical pathway from reversible to irreversible amyloid aggregation, we found that familial ALS variants of TDP-43 convert LARKS to irreversible aggregates. Our structures suggest how TDP-43 adopts both reversible and irreversible β-sheet aggregates and the role of mutation in the possible transition of reversible to irreversible pathogenic aggregation.
Organizational Affiliation:
Howard Hughes Medical Institute, University of California, Los Angeles, Los Angeles, CA, USA.