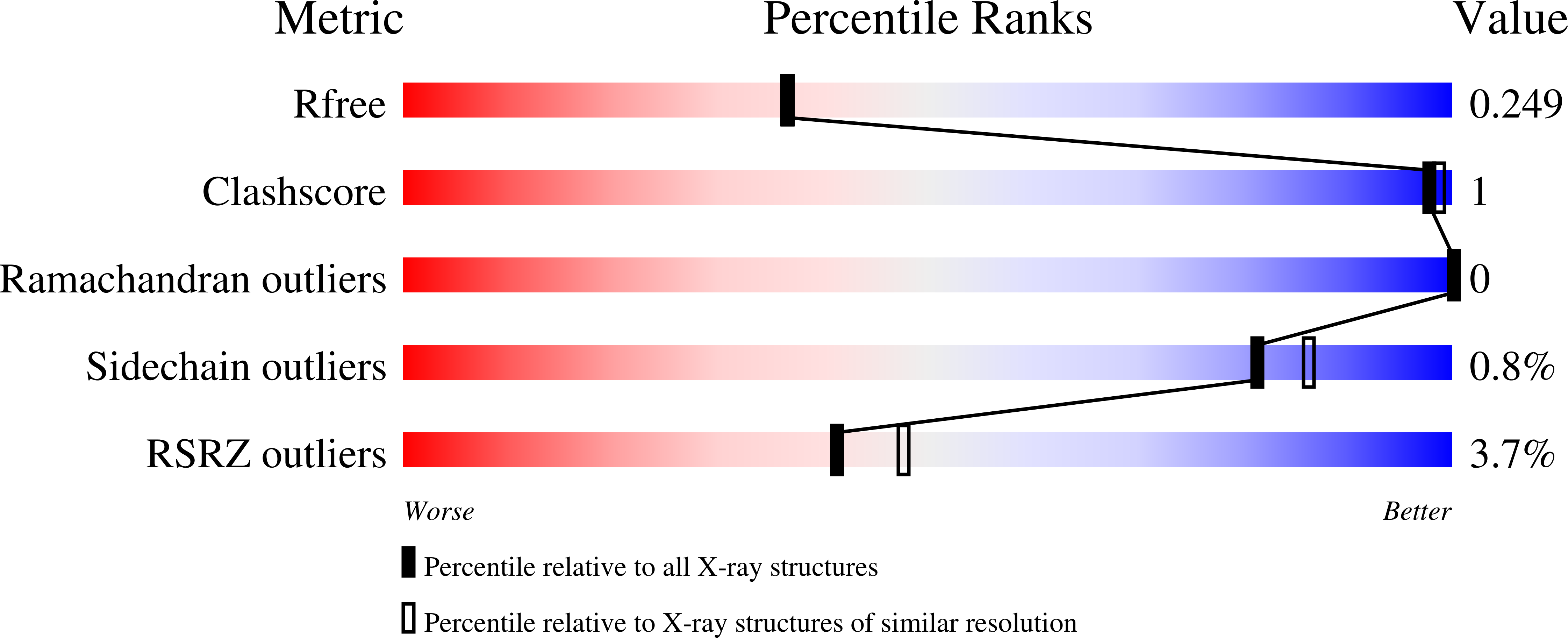
A Toxoplasma gondii locus required for the direct manipulation of host mitochondria has maintained multiple ancestral functions.
Blank, M.L., Parker, M.L., Ramaswamy, R., Powell, C.J., English, E.D., Adomako-Ankomah, Y., Pernas, L.F., Workman, S.D., Boothroyd, J.C., Boulanger, M.J., Boyle, J.P.(2018) Mol Microbiol 108: 519-535
- PubMed: 29505111
- DOI: https://doi.org/10.1111/mmi.13947
- Primary Citation of Related Structures:
6BXR, 6BXS, 6BXT, 6BXW - PubMed Abstract:
The Toxoplasma gondii locus mitochondrial association factor 1 (MAF1) encodes multiple paralogs, some of which mediate host mitochondrial association (HMA). Previous work showed that HMA was a trait that arose in T. gondii through neofunctionalization of an ancestral MAF1 ortholog. Structural analysis of HMA-competent and incompetent MAF1 paralogs (MAF1b and MAF1a, respectively) revealed that both paralogs harbor an ADP ribose binding macro-domain, with comparatively low (micromolar) affinity for ADP ribose. Replacing the 16 C-terminal residues of MAF1b with those of MAF1a abrogated HMA, and we also show that only three residues in the C-terminal helix are required for MAF1-mediated HMA. Importantly these same three residues are also required for the in vivo growth advantage conferred by MAF1b, providing a definitive link between in vivo proliferation and manipulation of host mitochondria. Co-immunoprecipitation assays reveal that the ability to interact with the mitochondrial MICOS complex is shared by HMA-competent and incompetent MAF1 paralogs and mutants. The weak ADPr coordination and ability to interact with the MICOS complex shared between divergent paralogs may represent modular ancestral functions for this tandemly expanded and diversified T. gondii locus.
Organizational Affiliation:
Department of Biological Sciences, University of Pittsburgh, Pittsburgh, PA, USA.