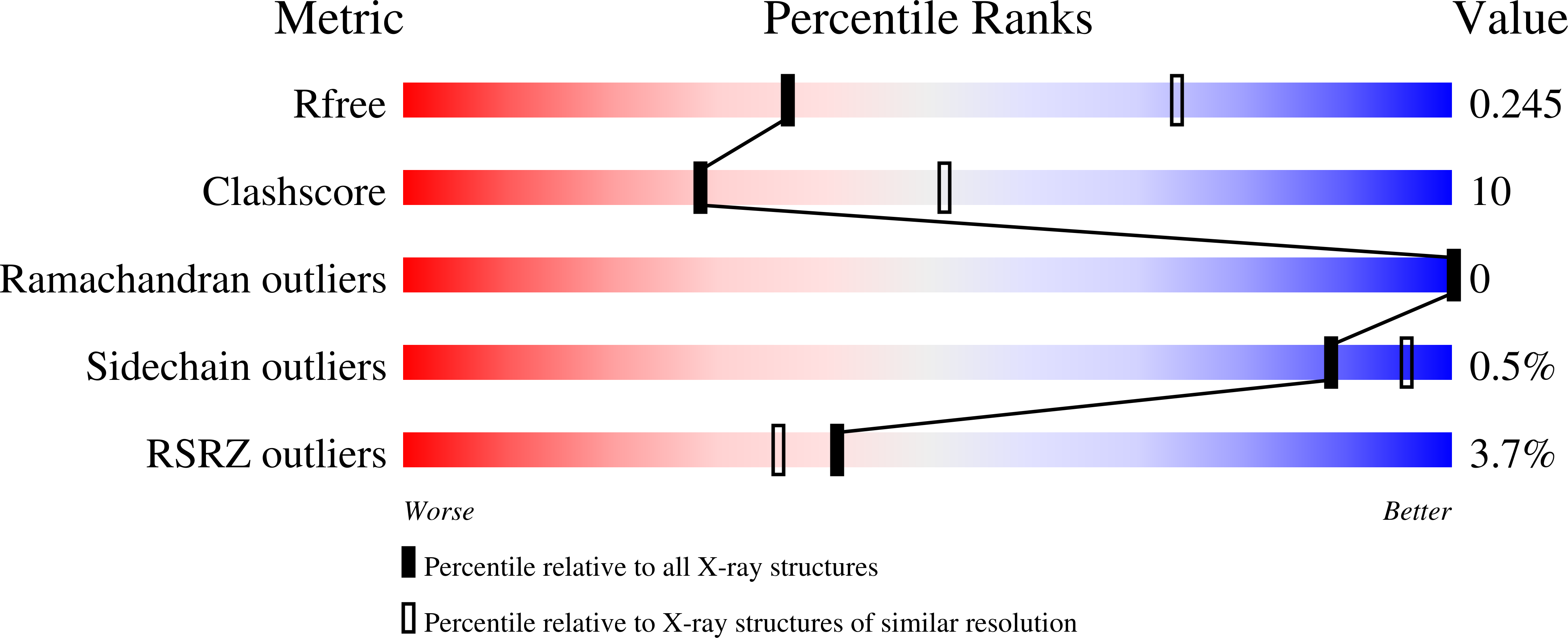
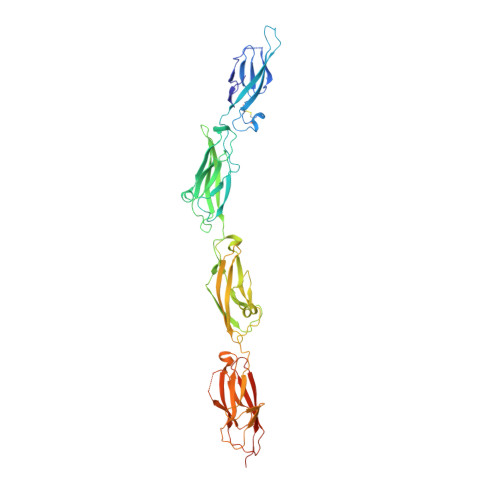
Identification of an adhesive interface for the non-clustered delta 1 protocadherin-1 involved in respiratory diseases.
Modak, D., Sotomayor, M.(2019) Commun Biol 2: 354-354
- PubMed: 31583286
- DOI: https://doi.org/10.1038/s42003-019-0586-0
- Primary Citation of Related Structures:
6BX7, 6MGA, 6PIM - PubMed Abstract:
Cadherins form a large family of calcium-dependent adhesive proteins involved in morphogenesis, cell differentiation, and neuronal connectivity. Non-clustered δ1 protocadherins form a cadherin subgroup of proteins with seven extracellular cadherin (EC) repeats and cytoplasmic domains distinct from those of classical cadherins. Non-clustered δ1 protocadherins mediate homophilic adhesion and have been implicated in various diseases including asthma, autism, and cancer. Here we present X-ray crystal structures of human Protocadherin-1 (PCDH1), a δ1-protocadherin member essential for New World Hantavirus infection that is typically expressed in the brain, airway epithelium, skin keratinocytes, and lungs. The structures suggest a binding mode that involves antiparallel overlap of repeats EC1 to EC4. Mutagenesis combined with binding assays and biochemical experiments validated this mode of adhesion. Overall, these results reveal the molecular mechanism underlying adhesiveness of PCDH1 and δ1-protocadherins, also shedding light on PCDH1's role in maintaining airway epithelial integrity, the loss of which causes respiratory diseases.
Organizational Affiliation:
Department of Chemistry and Biochemistry, The Ohio State University, 484 W 12th Avenue, Columbus, OH 43210 USA.