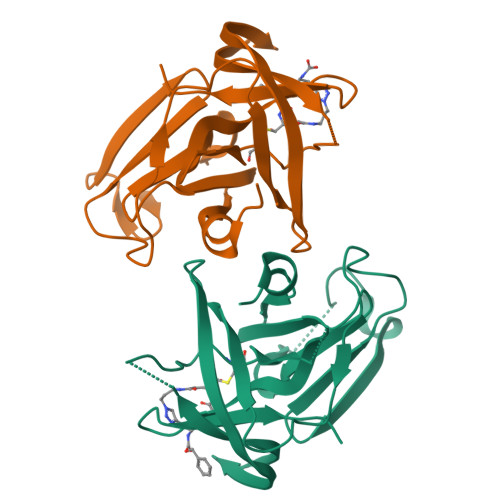
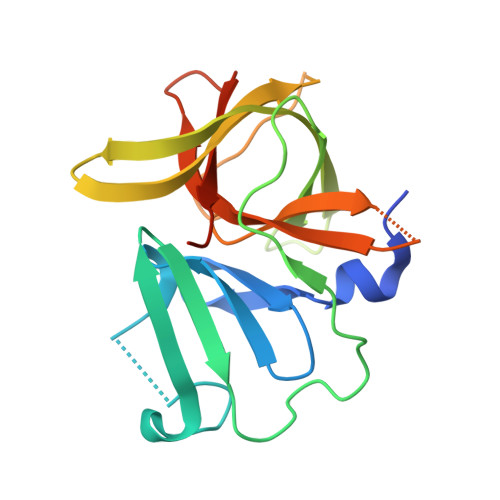
Putative structural rearrangements associated with the interaction of macrocyclic inhibitors with norovirus 3CL protease.
Galasiti Kankanamalage, A.C., Weerawarna, P.M., Rathnayake, A.D., Kim, Y., Mehzabeen, N., Battaile, K.P., Lovell, S., Chang, K.O., Groutas, W.C.(2019) Proteins 87: 579-587
- PubMed: 30883881
- DOI: https://doi.org/10.1002/prot.25682
- Primary Citation of Related Structures:
6BIB, 6BIC, 6BID - PubMed Abstract:
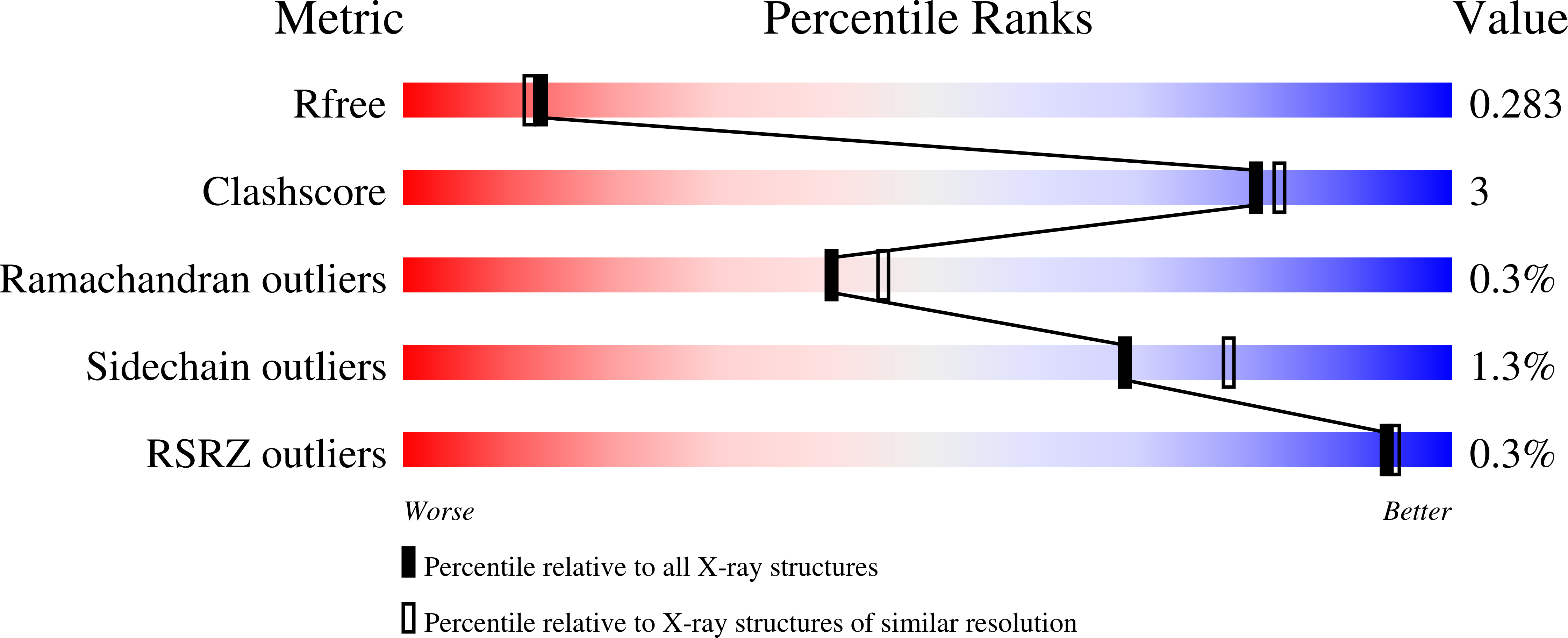
Human noroviruses are the primary cause of outbreaks of acute gastroenteritis worldwide. The problem is further compounded by the current lack of norovirus-specific antivirals or vaccines. Noroviruses have a single-stranded, positive sense 7 to 8 kb RNA genome which encodes a polyprotein precursor that is processed by a virus-encoded 3C-like cysteine protease (NV 3CLpro) to generate at least six mature nonstructural proteins. Processing of the polyprotein is essential for virus replication, consequently, NV 3CLpro has emerged as an attractive target for the discovery of norovirus therapeutics and prophylactics. We have recently described the structure-based design of macrocyclic transition state inhibitors of NV 3CLpro. In order to gain insight and understanding into the interaction of macrocyclic inhibitors with the enzyme, as well as probe the effect of ring size on pharmacological activity and cellular permeability, additional macrocyclic inhibitors were synthesized and high resolution cocrystal structures determined. The results of our studies tentatively suggest that the macrocyclic scaffold may hamper optimal binding to the active site by impeding concerted cross-talk between the S 2 and S 4 subsites.
Organizational Affiliation:
Department of Chemistry, Wichita State University, Kansas.