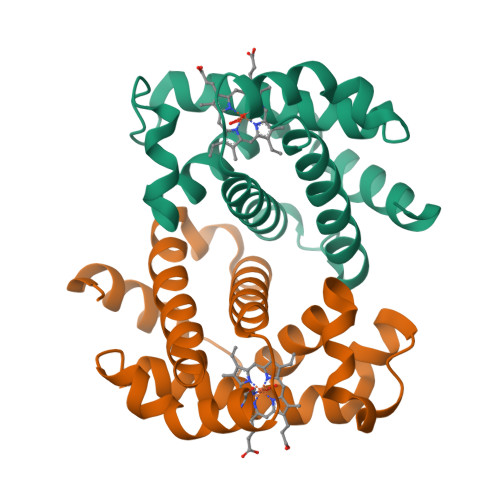
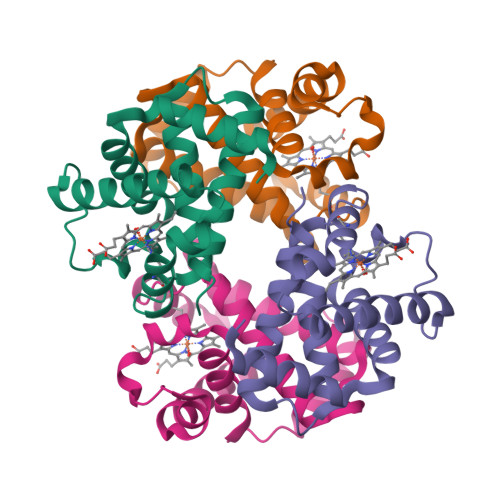
Hemoglobin crystals immersed in liquid oxygen reveal diffusion channels.
Terrell, J.R., Gumpper, R.H., Luo, M.(2018) Biochem Biophys Res Commun 495: 1858-1863
- PubMed: 29246762
- DOI: https://doi.org/10.1016/j.bbrc.2017.12.038
- Primary Citation of Related Structures:
5WOG, 5WOH, 6BB5 - PubMed Abstract:
Human hemoglobin (HbA) transports molecular oxygen (O 2 ) from the lung to tissues where the partial pressure of O 2 is lower. O 2 binds to HbA at the heme cofactor and is stabilized by a distal histidine (HisE7). HisE7 has been observed to occupy opened and closed conformations, and is postulated to act as a gate controlling the binding/release of O 2 . However, it has been suggested that HbA also contains intraprotein oxygen channels for entrances/exits far from the heme. In this study, we developed a novel method of crystal immersion in liquid oxygen prior to X-ray data collection. In the crystals immersed in liquid oxygen, the heme center was oxidized to generate aquomethemoglobin. Increases of structural flexibility were also observed in regions that are synonymous with previously postulated oxygen channels. These regions also correspond to medically relevant mutations which affect O 2 affinity. The way HbA utilizes these O 2 channels could have a profound impact on understanding the relationship of HbA O 2 transport within these disease conditions. Finally, the liquid oxygen immersion technique can be utilized as a new tool to crystallographically examine proteins and protein complexes which utilize O 2 for enzyme catalysis or transport.
Organizational Affiliation:
Department of Chemistry, Georgia State University, Atlanta, GA 30302, USA; Center for Diagnostics and Therapeutics, Georgia State University, Atlanta, GA 30302, USA.