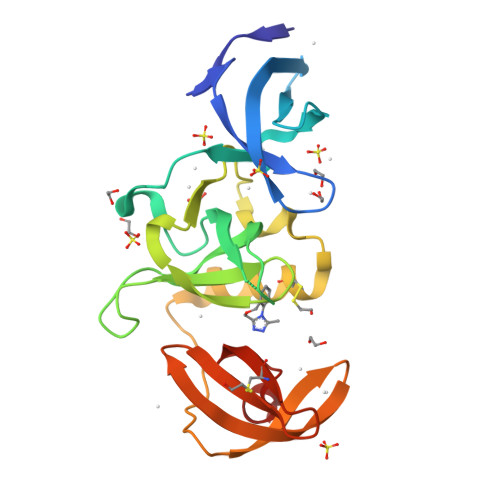
Identification and characterization of the first fragment hits for SETDB1 Tudor domain.
Mader, P., Mendoza-Sanchez, R., Iqbal, A., Dong, A., Dobrovetsky, E., Corless, V.B., Liew, S.K., Houliston, S.R., De Freitas, R.F., Smil, D., Sena, C.C.D., Kennedy, S., Diaz, D.B., Wu, H., Dombrovski, L., Allali-Hassani, A., Min, J., Schapira, M., Vedadi, M., Brown, P.J., Santhakumar, V., Yudin, A.K., Arrowsmith, C.H.(2019) Bioorg Med Chem 27: 3866-3878
- PubMed: 31327677
- DOI: https://doi.org/10.1016/j.bmc.2019.07.020
- Primary Citation of Related Structures:
6AU2 - PubMed Abstract:
SET domain bifurcated protein 1 (SETDB1) is a human histone-lysine methyltransferase which is amplified in human cancers and was shown to be crucial in the growth of non-small and small cell lung carcinoma. In addition to its catalytic domain, SETDB1 harbors a unique tandem tudor domain which recognizes histone sequences containing both methylated and acetylated lysines, and likely contributes to its localization on chromatin. Using X-ray crystallography and NMR spectroscopy fragment screening approaches, we have identified the first small molecule fragment hits that bind to histone peptide binding groove of the Tandem Tudor Domain (TTD) of SETDB1. Herein, we describe the binding modes of these fragments and analogues and the biophysical characterization of key compounds. These confirmed small molecule fragments will inform the development of potent antagonists of SETDB1 interaction with histones.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto, Toronto, Canada.