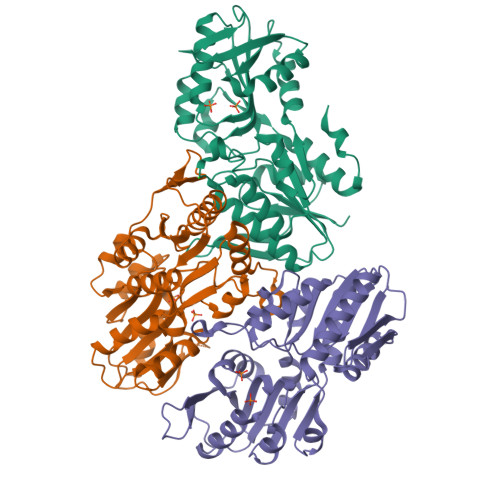
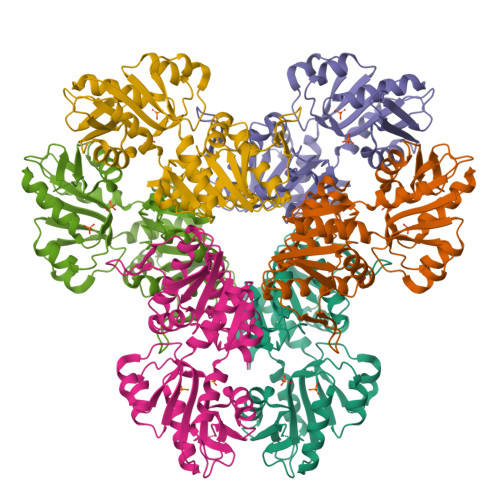
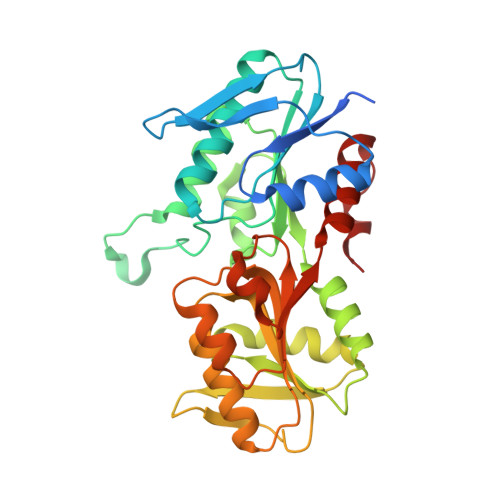
Crystal structure of E. coli PRPP synthetase.
Zhou, W., Tsai, A., Dattmore, D.A., Stives, D.P., Chitrakar, I., D'alessandro, A.M., Patil, S., Hicks, K.A., French, J.B.(2019) BMC Struct Biol 19: 1-1
- PubMed: 30646888
- DOI: https://doi.org/10.1186/s12900-019-0100-4
- Primary Citation of Related Structures:
6ASV - PubMed Abstract:
Ribose-phosphate pyrophosphokinase (EC 2.7.6.1) is an enzyme that catalyzes the ATP-dependent conversion of ribose-5-phosphate to phosphoribosyl pyrophosphate. The reaction product is a key precursor for the biosynthesis of purine and pyrimidine nucleotides. We report the 2.2 Å crystal structure of the E. coli ribose-phosphate pyrophosphobinase (EcKPRS). The protein has two type I phosphoribosyltransferase folds, related by 2-fold pseudosymmetry. The propeller-shaped homohexameric structure of KPRS is composed of a trimer of dimers, with the C-terminal domains forming the dimeric blades of the propeller and the N-terminal domains forming the hexameric core. The key, conserved active site residues are well-defined in the structure and positioned appropriately to bind substrates, adenosine monophosphate and ribose-5-phosphate. The allosteric site is also relatively well conserved but, in the EcKPRS structure, several residues from a flexible loop occupy the site where the allosteric modulator, adenosine diphosphate, is predicted to bind. The presence of the loop in the allosteric site may be an additional level of regulation, whereby low affinity molecules are precluded from binding. Overall, this study details key structural features of an enzyme that catalyzes a critical step in nucleotide metabolism. This work provides a framework for future studies of this important protein and, as nucleotides are critical for viability, may serve as a foundation for the development of novel anti-bacterial drugs.
Organizational Affiliation:
Department of Chemistry, Stony Brook University, Stony Brook, NY, 11794, USA.