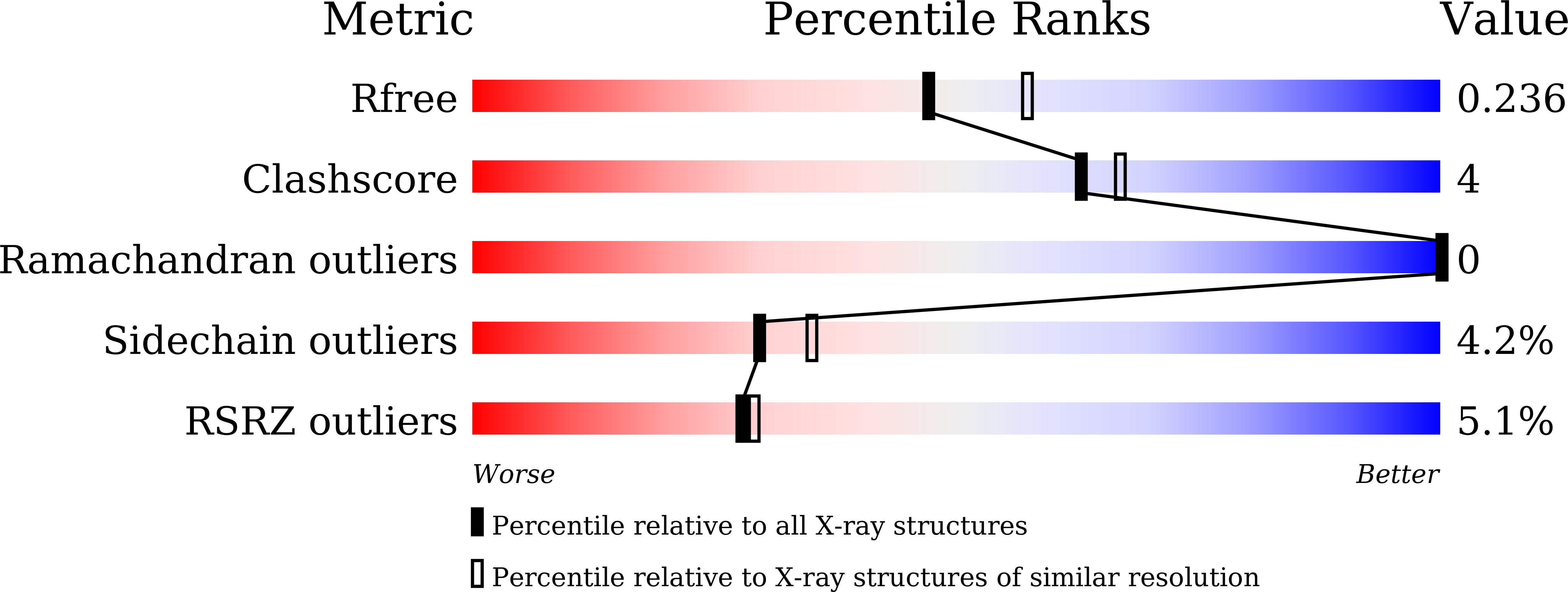
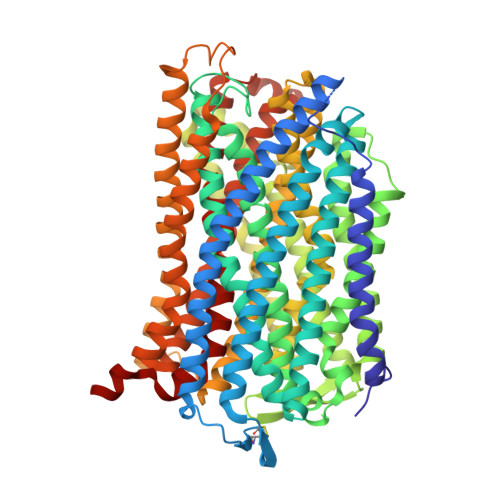
Roles of the Hydrophobic Gate and Exit Channel in Vigna radiata Pyrophosphatase Ion Translocation.
Tsai, J.Y., Tang, K.Z., Li, K.M., Hsu, B.L., Chiang, Y.W., Goldman, A., Sun, Y.J.(2019) J Mol Biol 431: 1619-1632
- PubMed: 30878480
- DOI: https://doi.org/10.1016/j.jmb.2019.03.009
- Primary Citation of Related Structures:
6AFS, 6AFT, 6AFU, 6AFV, 6AFW, 6AFX, 6AFY, 6AFZ - PubMed Abstract:
Membrane-embedded pyrophosphatase (M-PPase) hydrolyzes pyrophosphate to drive ion (H + and/or Na + ) translocation. We determined crystal structures and functions of Vigna radiata M-PPase (VrH + -PPase), the VrH + -PPase-2P i complex and mutants at hydrophobic gate (residue L555) and exit channel (residues T228 and E225). Ion pore diameters along the translocation pathway of three VrH + -PPases complexes (P i -, 2P i - and imidodiphosphate-bound states) present a unique wave-like profile, with different pore diameters at the hydrophobic gate and exit channel, indicating that the ligands induced pore size alterations. The 2P i -bound state with the largest pore diameter might mimic the hydrophobic gate open. In mutant structures, ordered waters detected at the hydrophobic gate among VrH + -PPase imply the possibility of solvation, and numerous waters at the exit channel might signify an open channel. A salt-bridge, E225-R562 is at the way out of the exit channel of VrH + -PPase; E225A mutant makes the interaction eliminated and reveals a decreased pumping ability. E225-R562 might act as a latch to regulate proton release. A water wire from the ion gate (R-D-K-E) through the hydrophobic gate and into the exit channel may reflect the path of proton transfer.
Organizational Affiliation:
Department of Life Science and Institute of Bioinformatics and Structural Biology, National Tsing Hua University, Hsinchu 30013, Taiwan.