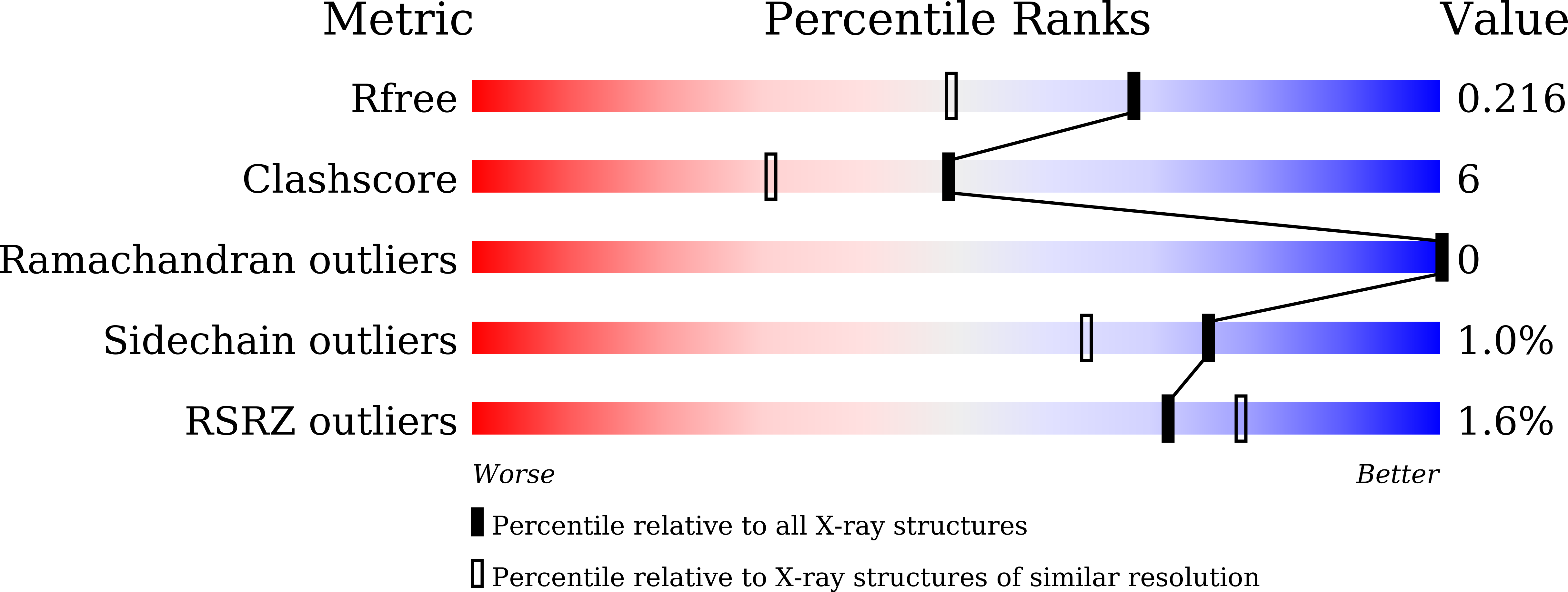
X-ray crystallographic analysis of time-dependent binding of guanidine hydrochloride to HEWL: First steps during protein unfolding.
Raskar, T., Koh, C.Y., Niebling, S., Kini, R.M., Hosur, M.V.(2019) Int J Biol Macromol 122: 903-913
- PubMed: 30412756
- DOI: https://doi.org/10.1016/j.ijbiomac.2018.11.023
- Primary Citation of Related Structures:
6A4N, 6A4O, 6A4P, 6A4Q - PubMed Abstract:
Time-dependent binding of guanidine hydrochloride (GuHCl) to hen egg-white lysozyme (HEWL), and effects of this binding on the protein structure have been investigated by solving X-ray structures of crystalline complexes. The complexes have been prepared by soaking, for different periods of time, native lysozyme crystals in solutions containing 2.5M GuHCl. In the refined structures, the number of water molecules in the protein's first solvent shell has progressively decreased from 152 to 115, showing protein's preference for guanidinium over water. Guanidinium ions preferentially hydrogen bond with the backbone carbonyl oxygen atoms. In their van der Waals interactions, they do not show any preference for apolar residues. Guanidinium ions have replaced water molecules that form cages around exposed hydrophobic residues. Guanidinium binding has decreased the average length of water-water hydrogen bond by 0.1Å. The hydrogen bonds between main chain atoms have been weakened by GuHCl, and this may be the reason for increased potency of GuHCl compared to urea. Guanidinium binding destabilizes the β-domain by causing loss of hydrogen bonds involving Asn 59 side chain. Interestingly, this loss is almost identical to that observed in structures of amyloidogenic variants of human lysozyme. Compounds preventing this loss could be anti-amyloidogenic.
Organizational Affiliation:
Ultrafast Molecular Dynamics Group, Centre for Hybrid Nanostructures (ChyN), University of Hamburg, Germany.