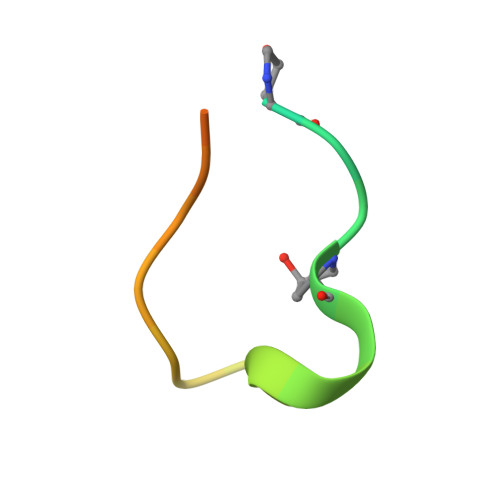
Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage.
Ge, C., Tong, D., Liang, B., Lonnblom, E., Schneider, N., Hagert, C., Viljanen, J., Ayoglu, B., Stawikowska, R., Nilsson, P., Fields, G.B., Skogh, T., Kastbom, A., Kihlberg, J., Burkhardt, H., Dobritzsch, D., Holmdahl, R.(2017) JCI Insight 2
- PubMed: 28679953
- DOI: https://doi.org/10.1172/jci.insight.93688
- Primary Citation of Related Structures:
5MU0, 5MU2, 5MUB, 5MV3, 5MV4 - PubMed Abstract:
Today, it is known that autoimmune diseases start a long time before clinical symptoms appear. Anti-citrullinated protein antibodies (ACPAs) appear many years before the clinical onset of rheumatoid arthritis (RA). However, it is still unclear if and how ACPAs are arthritogenic. To better understand the molecular basis of pathogenicity of ACPAs, we investigated autoantibodies reactive against the C1 epitope of collagen type II (CII) and its citrullinated variants. We found that these antibodies are commonly occurring in RA. A mAb (ACC1) against citrullinated C1 was found to cross-react with several noncitrullinated epitopes on native CII, causing proteoglycan depletion of cartilage and severe arthritis in mice. Structural studies by X-ray crystallography showed that such recognition is governed by a shared structural motif "RG-TG" within all the epitopes, including electrostatic potential-controlled citrulline specificity. Overall, we have demonstrated a molecular mechanism that explains how ACPAs trigger arthritis.
- Section for Medical Inflammation Research, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden.
Organizational Affiliation: