aKMT Catalyzes Extensive Protein Lysine Methylation in the Hyperthermophilic Archaeon Sulfolobus islandicus but is Dispensable for the Growth of the Organism
Chu, Y., Zhu, Y., Chen, Y., Li, W., Zhang, Z., Liu, D., Wang, T., Ma, J., Deng, H., Liu, Z.J., Ouyang, S., Huang, L.(2016) Mol Cell Proteomics 15: 2908-2923
- PubMed: 27329856
- DOI: https://doi.org/10.1074/mcp.M115.057778
- Primary Citation of Related Structures:
5FA8, 5FAD - PubMed Abstract:
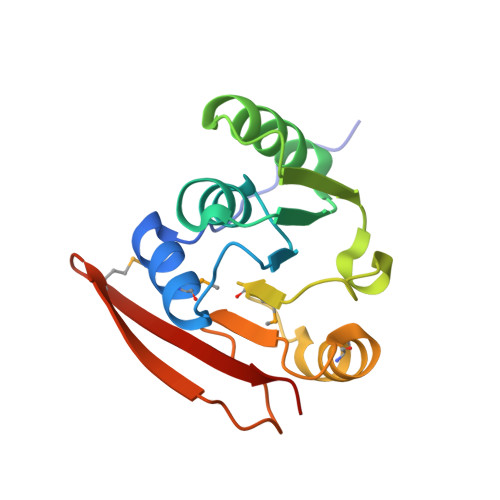
Protein methylation is believed to occur extensively in creanarchaea. Recently, aKMT, a highly conserved crenarchaeal protein lysine methyltransferase, was identified and shown to exhibit broad substrate specificity in vitro Here, we have constructed an aKMT deletion mutant of the hyperthermophilic crenarchaeon Sulfolobus islandicus The mutant was viable but showed a moderately slower growth rate than the parental strain under non-optimal growth conditions. Consistent with the moderate effect of the lack of aKMT on the growth of the cell, expression of a small number of genes, which encode putative functions in substrate transportation, energy metabolism, transcriptional regulation, stress response proteins, etc, was differentially regulated by more than twofold in the mutant strain, as compared with that in the parental strain. Analysis of the methylation of total cellular protein by mass spectrometry revealed that methylated proteins accounted for ∼2/3 (1,158/1,751) and ∼1/3 (591/1,757) of the identified proteins in the parental and the mutant strains, respectively, indicating that there is extensive protein methylation in S. islandicus and that aKMT is a major protein methyltransferase in this organism. No significant sequence preference was detected at the sites of methylation by aKMT. Methylated lysine residues, when visible in the structure, are all located on the surface of the proteins. The crystal structure of aKMT in complex with S-adenosyl-l-methionine (SAM) or S-adenosyl homocysteine (SAH) reveals that the protein consists of four α helices and seven β sheets, lacking a substrate recognition domain found in PrmA, a bacterial homolog of aKMT, in agreement with the broad substrate specificity of aKMT. Our results suggest that aKMT may serve a role in maintaining the methylation status of cellular proteins required for the efficient growth of the organism under certain non-optimal conditions.
- From the ‡State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China;
Organizational Affiliation: