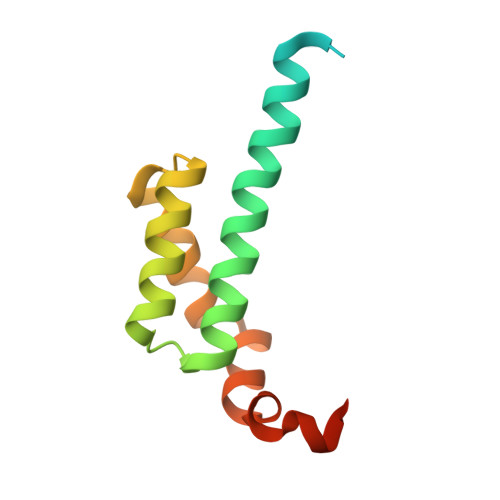
Crystal structure of an ENT domain from Trypanosoma brucei.
Mi, J., Yang, X., Zhang, J., Zhang, X., Xu, C., Liao, S., Tu, X.(2018) Biochem Biophys Res Commun 505: 755-760
- PubMed: 30293681
- DOI: https://doi.org/10.1016/j.bbrc.2018.09.167
- Primary Citation of Related Structures:
5ZX1 - PubMed Abstract:
Trypanosoma brucei (T. brucei) is a parasitic protozoan causing human sleeping sickness and related animal diseases. ENT (EMSY N-terminal) domain was first found in EMSY protein which has been proved to be involved in multiple biological processes such as DNA repair, tumorigenesis, and transcriptional regulation. So far, little is known about the function and structure of ENT domains from protozoan. Q385P5 from T. brucei, containing an ENT domain at its N-terminus, is a conserved protein in related kinetoplastid parasites. In this work, the crystal structure of ENT domain of Q385P5 (TbENT) was solved at a resolution of 2.3 Å. TbENT adopts a club-like shape consisting of five helixes, which is similar to the structure of human EMSY ENT domain (HsENT). Interestingly, TbENT shows significantly different orientation on the fifth α-helix compared with HsENT. Meanwhile, human HP1 interacts with a conserved motif adjacent to EMSY ENT domain. However, this conserved binding motif is absent in Q385P5. These differences may imply the different protein interactions and roles of Q385P5 and its ENT domain in T. brucei.
Organizational Affiliation:
Hefei National Laboratory for Physical Science at Microscale and School of Life Science, University of Science and Technology of China, Hefei, Anhui, 230026, PR China.