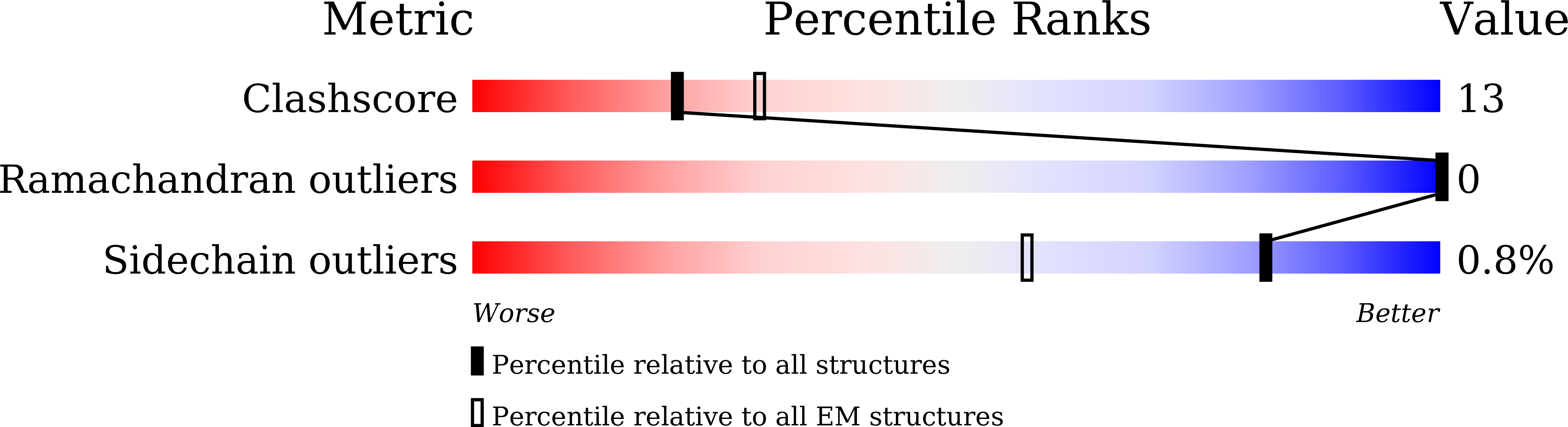Cryo-EM structures of the human volume-regulated anion channel LRRC8.
Kasuya, G., Nakane, T., Yokoyama, T., Jia, Y., Inoue, M., Watanabe, K., Nakamura, R., Nishizawa, T., Kusakizako, T., Tsutsumi, A., Yanagisawa, H., Dohmae, N., Hattori, M., Ichijo, H., Yan, Z., Kikkawa, M., Shirouzu, M., Ishitani, R., Nureki, O.(2018) Nat Struct Mol Biol 25: 797-804
- PubMed: 30127360
- DOI: https://doi.org/10.1038/s41594-018-0109-6
- Primary Citation of Related Structures:
5ZSU - PubMed Abstract:
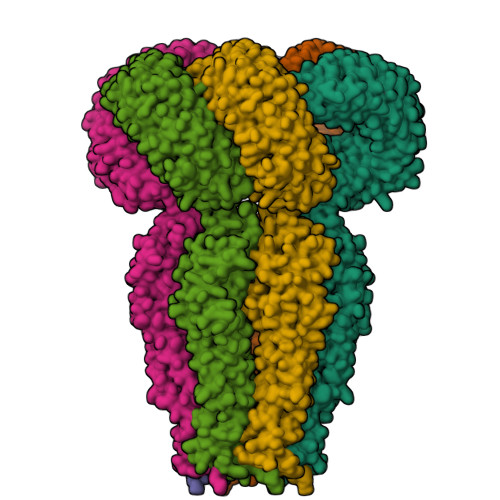
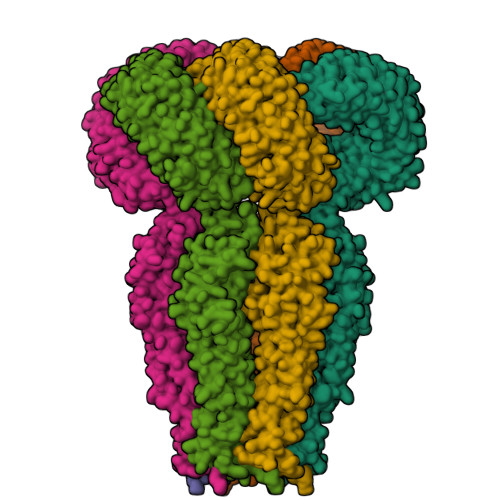
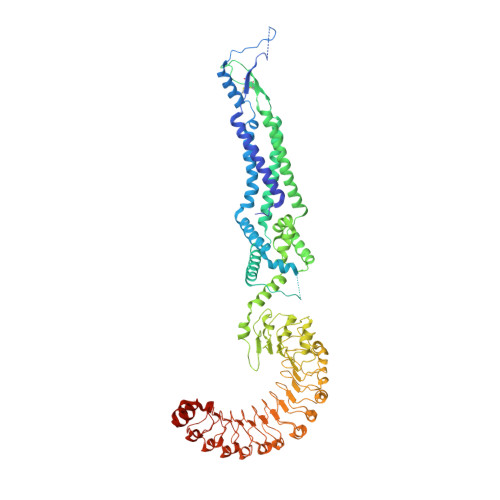
Maintenance of cell volume against osmotic change is crucial for proper cell functions. Leucine-rich repeat-containing 8 proteins are anion-selective channels that extrude anions to decrease the cell volume on cellular swelling. Here, we present the structure of human leucine-rich repeat-containing 8A, determined by single-particle cryo-electron microscopy. The structure shows a hexameric assembly, and the transmembrane region features a topology similar to gap junction channels. The LRR region, with 15 leucine-rich repeats, forms a long, twisted arc. The channel pore is located along the central axis and constricted on the extracellular side, where highly conserved polar and charged residues at the tip of the extracellular helix contribute to permeability to anions and other osmolytes. Two structural populations were identified, corresponding to compact and relaxed conformations. Comparing the two conformations suggests that the LRR region is flexible and mobile, with rigid-body motions, which might be implicated in structural transitions on pore opening.
Organizational Affiliation:
Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Bunkyo-ku, Tokyo, Japan.