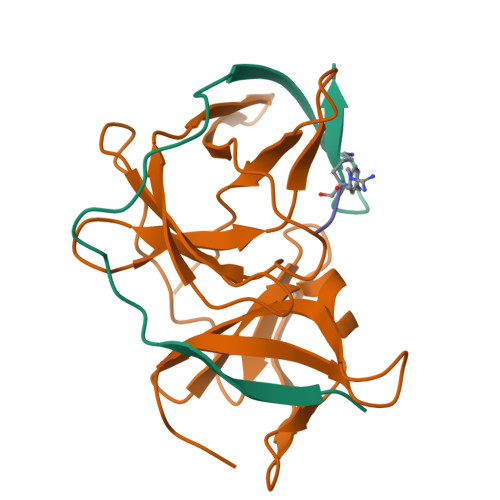
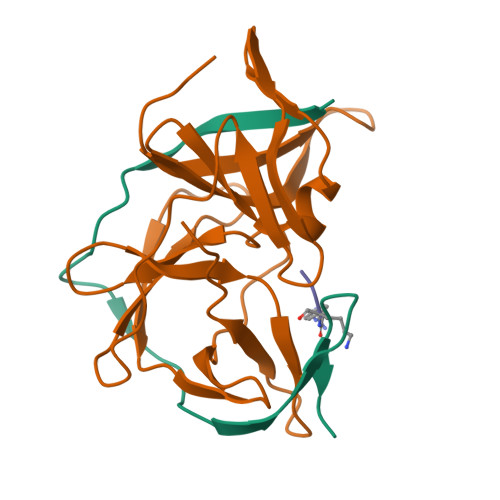
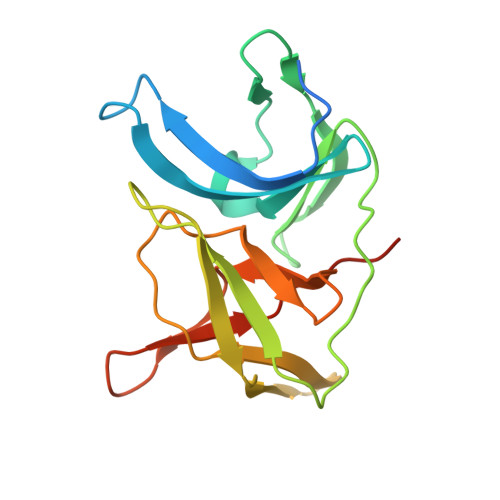
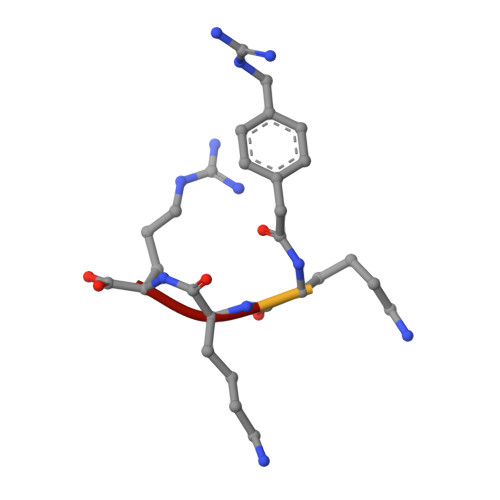
Structures of Zika virus NS2B-NS3 protease in complex with peptidomimetic inhibitors.
Phoo, W.W., Zhang, Z., Wirawan, M., Chew, E.J.C., Chew, A.B.L., Kouretova, J., Steinmetzer, T., Luo, D.(2018) Antiviral Res 160: 17-24
- PubMed: 30315877
- DOI: https://doi.org/10.1016/j.antiviral.2018.10.006
- Primary Citation of Related Structures:
5ZMQ, 5ZMS, 5ZOB - PubMed Abstract:
Zika virus NS2B-NS3 protease plays an essential role in viral replication by processing the viral polyprotein into individual proteins. The viral protease is therefore considered as an ideal antiviral drug target. To facilitate the development of protease inhibitors, we report three high-resolution co-crystal structures of bZiPro with peptidomimetic inhibitors composed of a P1-P4 segment and different P1' residues. Compounds 1 and 2 possess small P1' groups that are split off by bZiPro, which could be detected by mass spectrometry. On the other hand, the more potent compound 3 contains a bulky P1' benzylamide structure that is resistant to cleavage by bZiPro, demonstrating that presence of an uncleavable C-terminal cap contributes to a slightly improved inhibitory potency. The N-terminal phenylacetyl residue occupies a position above the P1 side chain and therefore stabilizes a horseshoe-like backbone conformation of the bound inhibitors. The P4 moieties show unique intra- and intermolecular interactions. Our work reports the detailed binding mode interactions of substrate-analogue inhibitors within the S4-S1' pockets and explains the preference of bZiPro for basic P1-P3 residues. These new structures of protease-inhibitor complexes will guide the design of more effective NS2B-NS3 protease inhibitors with improved potency and bioavailability.
Organizational Affiliation:
Lee Kong Chian School of Medicine, Nanyang Technological University, EMB 03-07, 59 Nanyang Drive, Singapore 636921, Singapore; NTU Institute of Structural Biology, Nanyang Technological University, EMB 06-01, 59 Nanyang Drive, Singapore 636921, Singapore; School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Singapore.