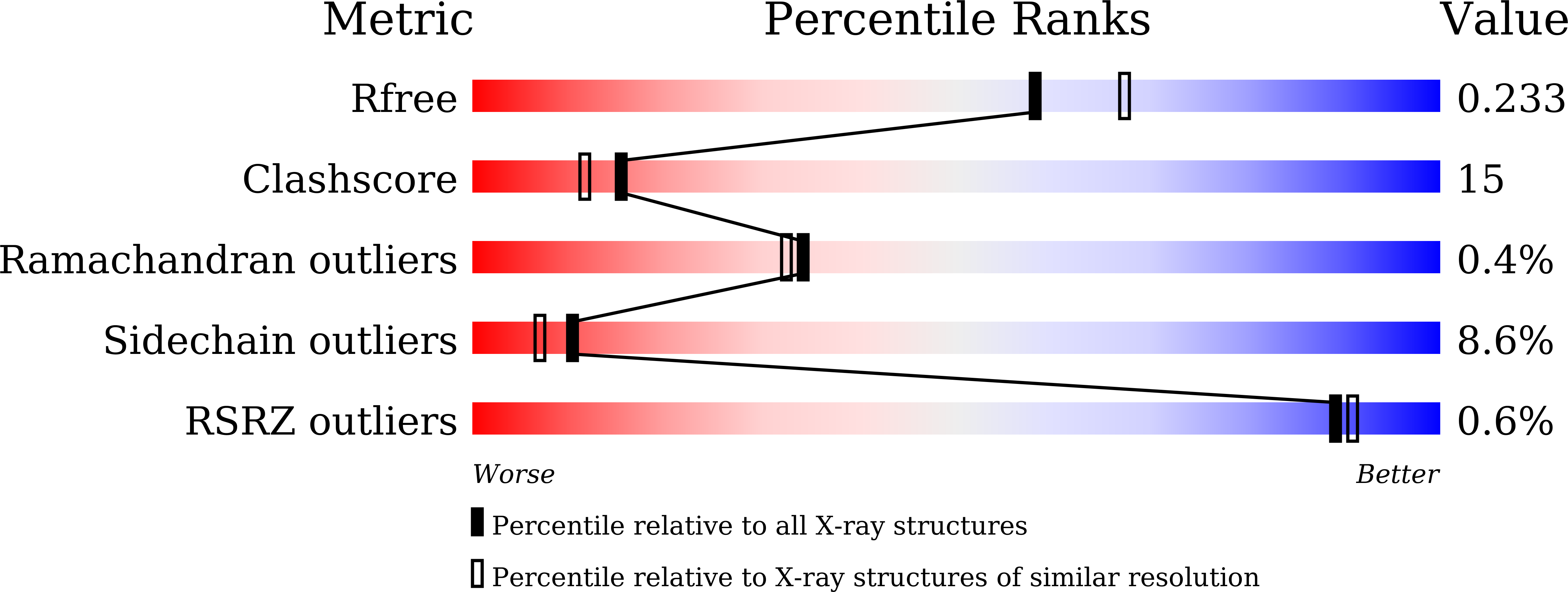
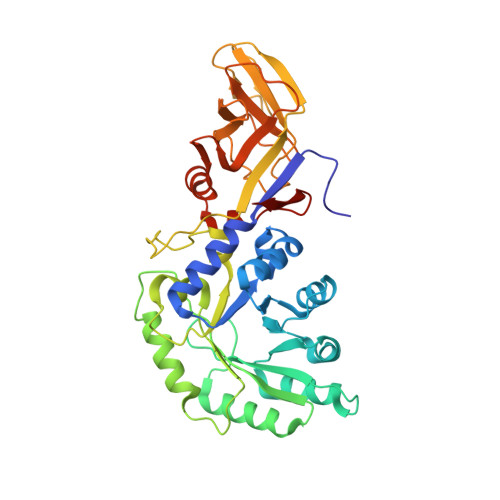
The first identification and characterization of a histidine-specific amino acid racemase, histidine racemase from a lactic acid bacterium, Leuconostoc mesenteroides subsp. sake NBRC 102480.
Adachi, M., Shimizu, R., Kato, S., Oikawa, T.(2019) Amino Acids 51: 331-343
- PubMed: 30377839
- DOI: https://doi.org/10.1007/s00726-018-2671-y
- Primary Citation of Related Structures:
5ZL6 - PubMed Abstract:
We expressed a histidine racemase from Leuconostoc mesenteroides subsp. sake NBRC 102480 (Lm-HisR) successively in a soluble fraction of Escherichia coli BL21 (DE3) and then highly purified it from the cell-free extract. Lm-HisR showed amino acid racemase activity on histidine specifically. This is the first example of an amino acid racemase specifically acting on histidine. Phylogenetic analysis of Lm-HisR showed that Lm-HisR was located far from the cluster of alanine racemases reported thus far and only in lactic acid bacteria of the genus Leuconostoc. Alignment of the primary structure of Lm-HisR with those of lysine and alanine racemases and alanine racemase homologs previously reported revealed that the PLP-binding lysine and catalytic tyrosine were completely conserved, and some residues that are unique to the phylogenetic branch of Lm-HisR, Phe44, Ser45, Thr174, Thr206, His286, Ser287, Phe292, Gly312, Val357, and Ala358 were identified. We determined the crystal structure of Lm-HisR complexed with PLP at a 2.1-Å resolution. The crystal structure contained four molecules (two dimers) in the asymmetric unit. When comparing the 3D structure of Lm-HisR with those of racemases from Geobacillus stearothermophilus and Oenococcus oeni, Met315 was completely conserved, but Val357 was not. In addition, two significant differences were observed between Lm-HisR and G. stearothermophilus alanine racemase. Phe44 and His286 in Lm-HisR corresponded to Tyr43 and Tyr284 in G. stearothermophilus alanine racemase, respectively. Based on the structural analysis, comparison with alanine racemase, and docking simulation, three significant residues, Phe44, His286, and Val357, were identified that may control the substrate specificity of Lm-HisR.
Organizational Affiliation:
Tokai Quantum Beam Science Center, Takasaki Advanced Radiation Research Institute, National Institutes for Quantum and Radiological Science and Technology, 2-4 Shirakata, Tokai, Ibaraki, 319-1106, Japan.