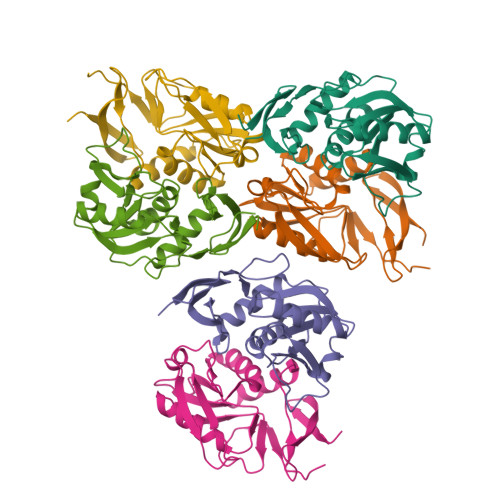
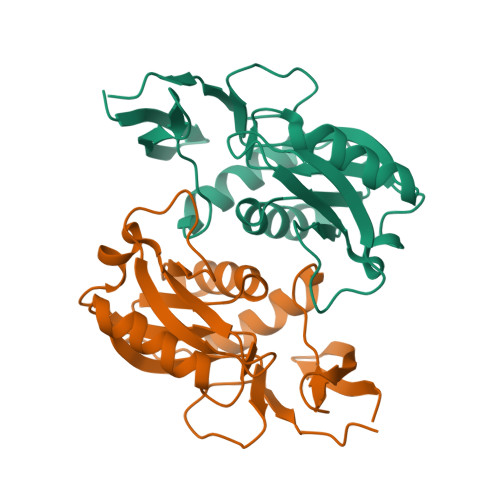
Structural and functional characterization of thermostable biocatalysts for the synthesis of 6-aminopurine nucleoside-5'-monophospate analogues.
Arco, J.D., Perez, E., Naitow, H., Matsuura, Y., Kunishima, N., Fernandez-Lucas, J.(2019) Bioresour Technol 276: 244-252
- PubMed: 30640018
- DOI: https://doi.org/10.1016/j.biortech.2018.12.120
- Primary Citation of Related Structures:
5ZGO - PubMed Abstract:
The present work describes the functional and structural characterization of adenine phosphoribosyltransferase 2 from Thermus thermophilus HB8 (TtAPRT2). The combination of structural and substrate specificity data provided valuable information for immobilization studies. Dimeric TtAPRT2 was immobilized onto glutaraldehyde-activated MagReSyn®Amine magnetic iron oxide porous microparticles by two different strategies: a) an enzyme immobilization at pH 8.5 to encourage the immobilization process by N-termini (MTtAPRT2A, MTtAPRT2B, MTtAPRT2C) or b) an enzyme immobilization at pH 10.0 to encourage the immobilization process through surface exposed lysine residues (MTtAPRT2D, MTtAPRT2E, MTtAPRT2F). According to catalyst load experiments, MTtAPRT2B (activity: 480 IU g -1 biocatalyst , activity recovery: 52%) and MTtAPRT2F (activity: 507 IU g -1 biocatalyst , activity recovery: 44%) were chosen as optimal derivatives. The biochemical characterization studies demonstrated that immobilization process improved the thermostability of TtAPRT2. Moreover, the potential reusability of MTtAPRT2B and MTtAPRT2F was also tested. Finally, MTtAPRT2F was employed in the synthesis of nucleoside-5'-monophosphate analogues.
Organizational Affiliation:
Applied Biotechnology Group, Biomedical Science School, Universidad Europea de Madrid, Urbanización El Bosque, Calle Tajo, s/n, 28670, Villaviciosa de Odón, Spain.