A Novel Orally Active Inverse Agonist of Estrogen-related Receptor Gamma (ERR gamma ), DN200434, A Booster of NIS in Anaplastic Thyroid Cancer.
Singh, T.D., Song, J., Kim, J., Chin, J., Ji, H.D., Lee, J.E., Lee, S.B., Yoon, H., Yu, J.H., Kim, S.K., Yoon, G.S., Hwang, H., Lee, H.W., Oh, J.M., Lee, S.W., Lee, J., Choi, H.S., Na, S.Y., Choi, W.I., Park, Y.J., Song, Y.S., Kim, Y.A., Lee, I.K., Cho, S.J., Jeon, Y.H.(2019) Clin Cancer Res 25: 5069-5081
- PubMed: 31010838
- DOI: https://doi.org/10.1158/1078-0432.CCR-18-3007
- Primary Citation of Related Structures:
5YSO - PubMed Abstract:
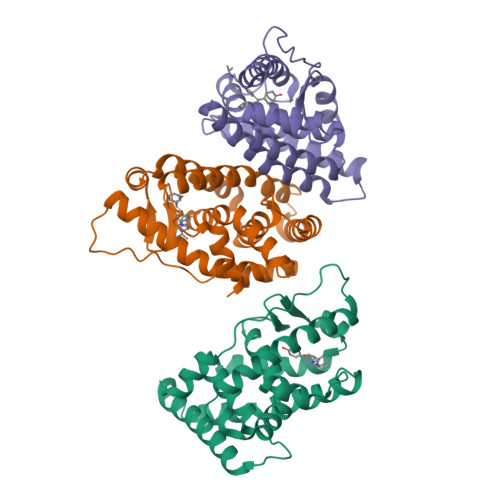
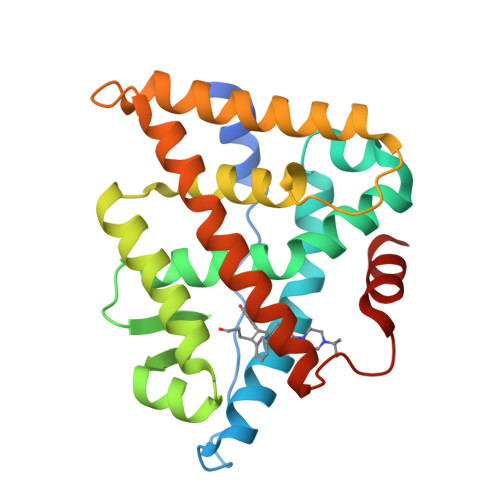
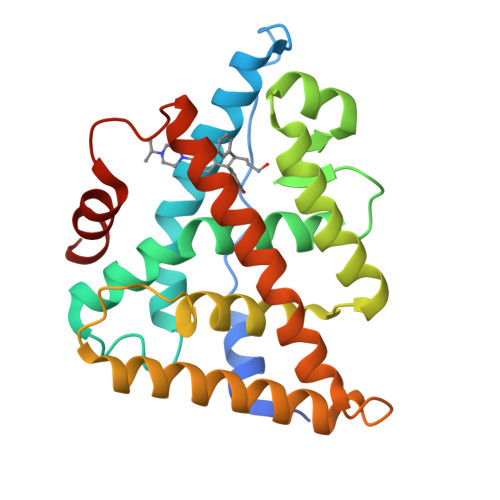
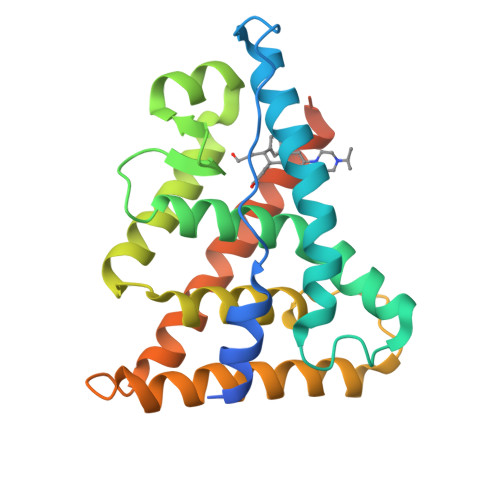
New strategies to restore sodium iodide symporter (NIS) expression and function in radioiodine therapy-refractive anaplastic thyroid cancers (ATCs) are urgently required. Recently, we reported the regulatory role of estrogen-related receptor gamma (ERRγ) in ATC cell NIS function. Herein, we identified DN200434 as a highly potent (functional IC 50 = 0.006 μmol/L), selective, and orally available ERRγ inverse agonist for NIS enhancement in ATC. We sought to identify better ERRγ-targeting ligands and explored the crystal structure of ERRγ in complex with DN200434. After treating ATC cells with DN200434, the change in iodide-handling gene expression, as well as radioiodine avidity was examined. ATC tumor-bearing mice were orally administered with DN200434, followed by 124 I-positron emission tomography/CT (PET/CT). For radioiodine therapy, ATC tumor-bearing mice treated with DN200434 were administered 131 I (beta ray-emitting therapeutic radioiodine) and then bioluminescent imaging was performed to monitor the therapeutic effects. Histologic analysis was performed to evaluate ERRγ expression status in normal tissue and ATC tissue, respectively. DN200434-ERRγ complex crystallographic studies revealed that DN200434 binds to key ERRγ binding pocket residues through four-way interactions. DN200434 effectively upregulated iodide-handling genes and restored radioiodine avidity in ATC tumor lesions, as confirmed by 124 I-PET/CT. DN200434 enhanced ATC tumor radioiodine therapy susceptibility, markedly inhibiting tumor growth. Histologic findings of patients with ATC showed higher ERRγ expression in tumors than in normal tissue, supporting ERRγ as a therapeutic target for ATC. DN200434 shows potential clinical applicability for diagnosis and treatment of ATC or other poorly differentiated thyroid cancers.
Organizational Affiliation:
Department of Medical Oncology Laboratory, All India Institute of Medical Sciences (AIIMS), New Delhi, India.