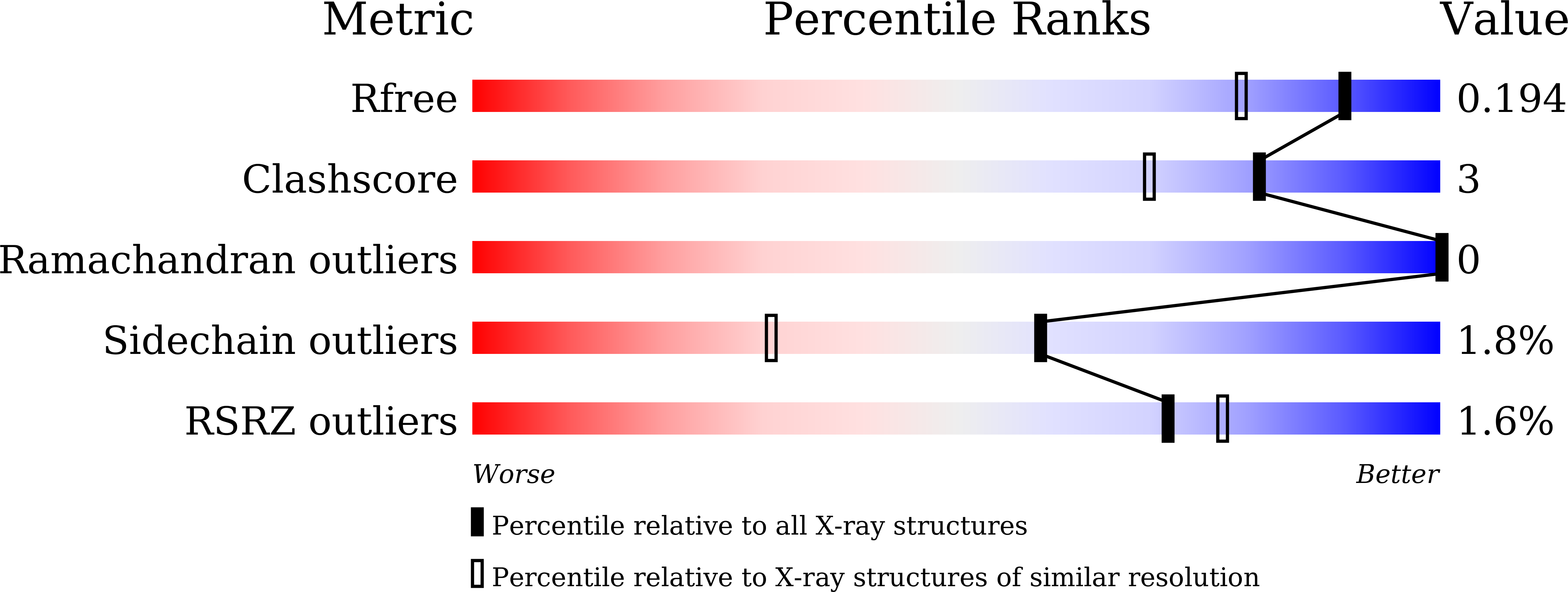
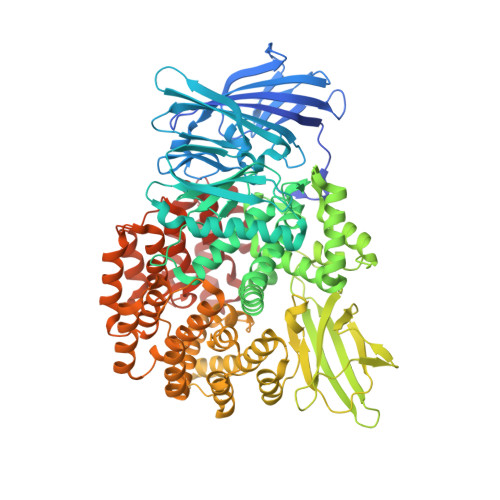
Puromycin, a selective inhibitor of PSA acts as a substrate for other M1 family aminopeptidases: Biochemical and structural basis
Reddi, R., Ganji, R.J., Marapaka, A.K., Bala, S.C., Yerra, N.V., Haque, N., Addlagatta, A.(2020) Int J Biol Macromol 165: 1373-1381