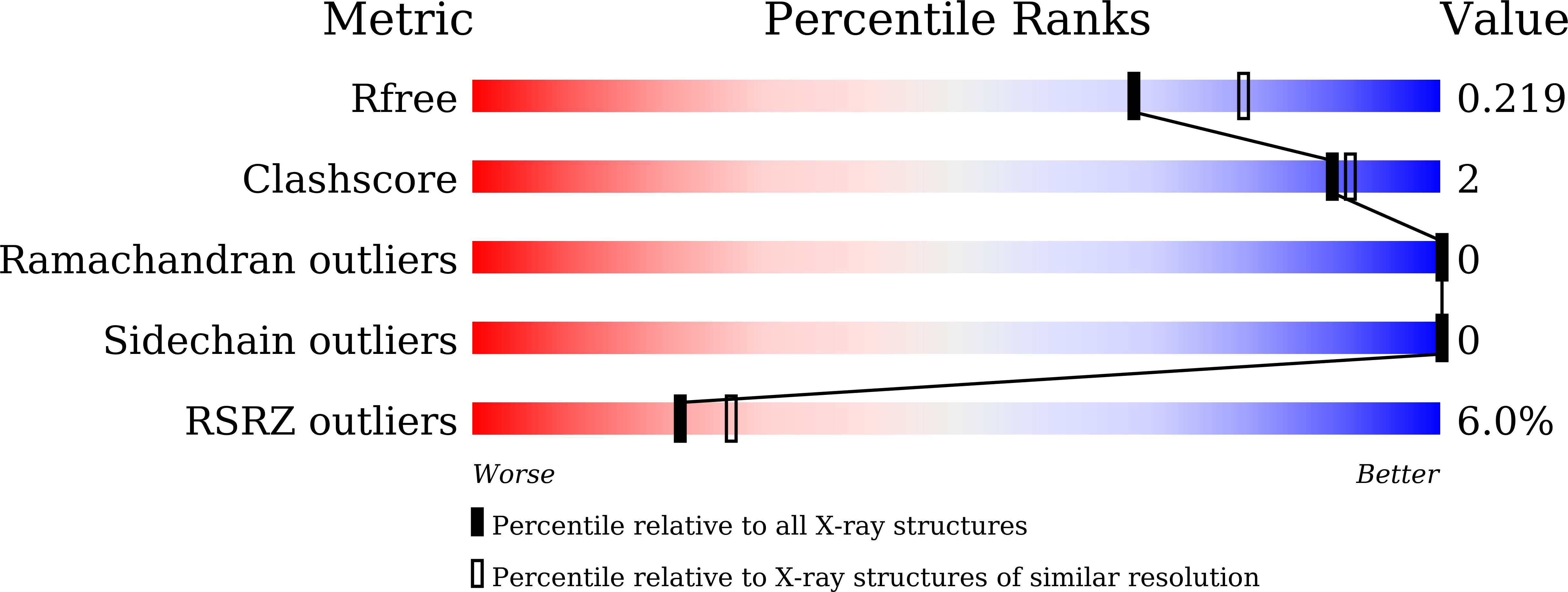
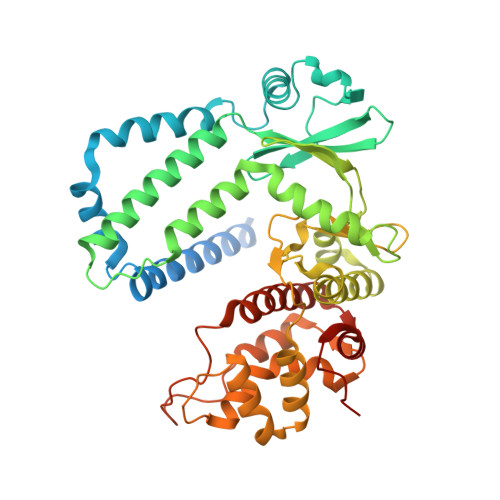
Discovery of the first macrolide antibiotic binding protein in Mycobacterium tuberculosis: a new antibiotic resistance drug target.
Zhang, Q., Liu, H., Liu, X., Jiang, D., Zhang, B., Tian, H., Yang, C., Guddat, L.W., Yang, H., Mi, K., Rao, Z.(2018) Protein Cell 9: 971-975