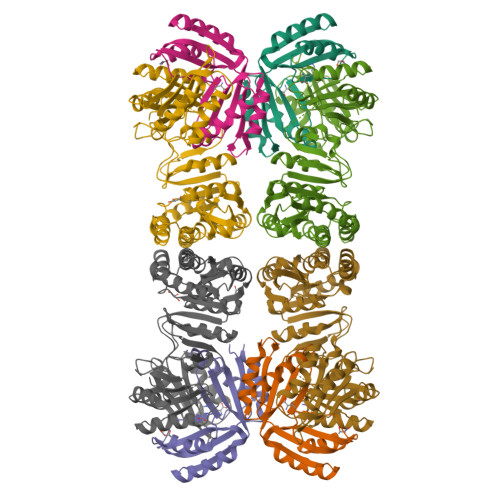
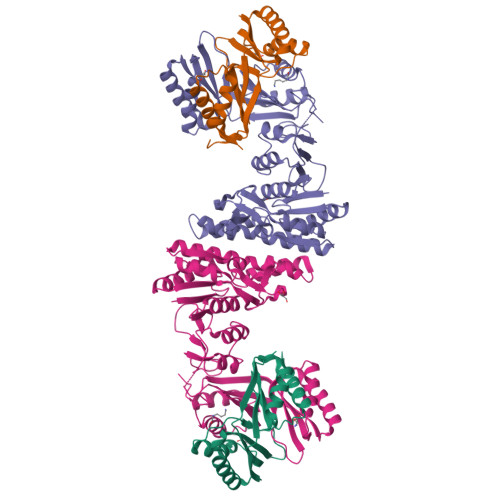
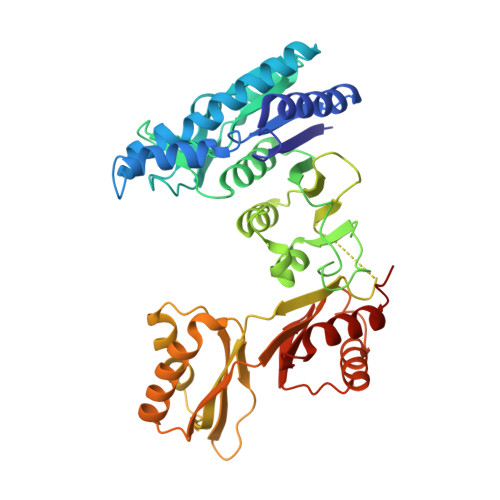
Mechanistic insights into the allosteric regulation of Pseudomonas aeruginosa aspartate kinase.
Li, C., Yang, M., Liu, L., Li, T., Peng, C., He, L., Song, Y., Zhu, Y., Shen, Y., Yang, J., Zhao, N., Zhao, C., Zhou, Q., Li, H., Kang, M., Tong, A., Tang, H., Bao, R.(2018) Biochem J 475: 1107-1119
- PubMed: 29382741
- DOI: https://doi.org/10.1042/BCJ20170829
- Primary Citation of Related Structures:
5YEI - PubMed Abstract:
In plants and microorganisms, aspartate kinase (AK) catalyzes an initial commitment step of the aspartate family amino acid biosynthesis. Owing to various structural organizations, AKs from different species show tremendous diversity and complex allosteric controls. We report the crystal structure of AK from Pseudomonas aeruginosa (PaAK), a typical α2β2 hetero-tetrameric enzyme, in complex with inhibitory effectors. Distinctive features of PaAK are revealed by structural and biochemical analyses. Essentially, the open conformation of Lys-/Thr-bound PaAK structure clarifies the inhibitory mechanism of α2β2-type AK. Moreover, the various inhibitory effectors of PaAK have been identified and a general amino acid effector motif of AK family is described.
Organizational Affiliation:
Center of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University and Collaborative Innovation Center, Chengdu, China.