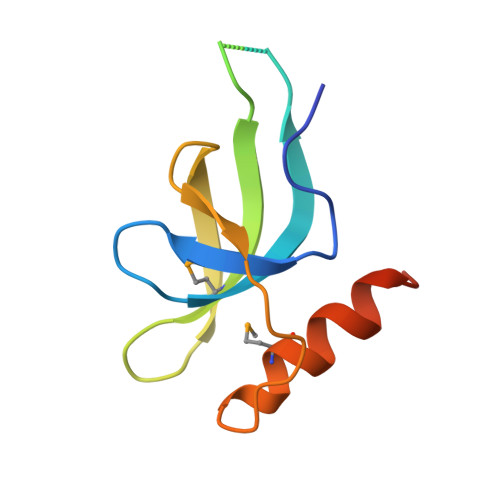
X-ray crystal structure of Escherichia coli HspQ, a protein involved in the retardation of replication initiation
Abe, Y., Shioi, S., Kita, S., Nakata, H., Maenaka, K., Kohda, D., Katayama, T., Ueda, T.(2017) FEBS Lett 591: 3805-3816
- PubMed: 29083032
- DOI: https://doi.org/10.1002/1873-3468.12892
- Primary Citation of Related Structures:
5YCQ - PubMed Abstract:
The heat shock protein HspQ (YccV) of Escherichia coli has been proposed to participate in the retardation of replication initiation in cells with the dnaA508 allele. In this study, we have determined the 2.5-Å-resolution X-ray structure of the trimer of HspQ, which is also the first structure of a member of the YccV superfamily. The acidic character of the HspQ trimer suggests an interaction surface with basic proteins. From these results, we discuss the cellular function of HspQ, including its relationship with the DnaA508 protein.
Organizational Affiliation:
Department of Protein Structure, Function and Design, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan.