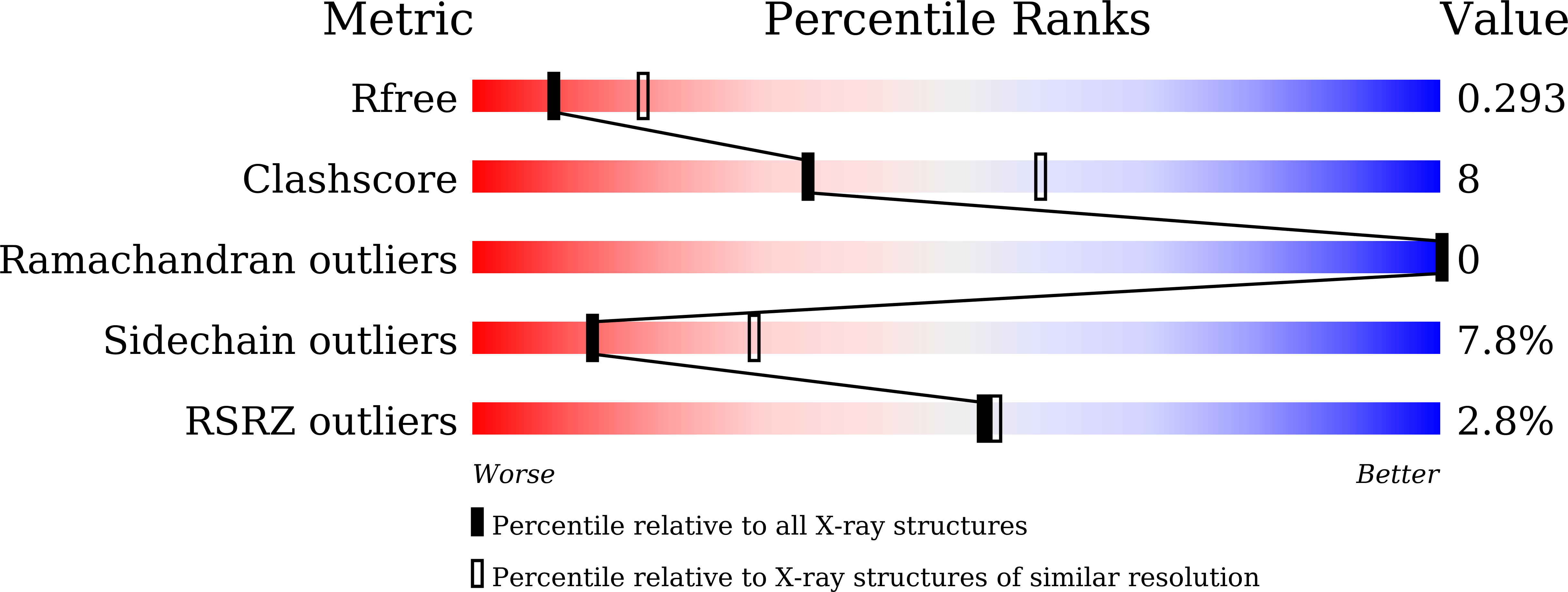
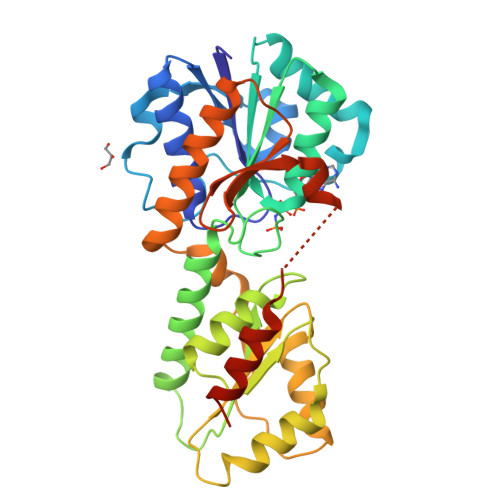
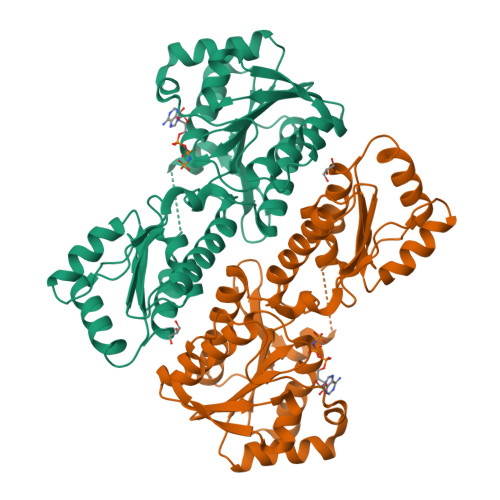
Structural Insights into the Regulation of Staphylococcus aureus Phosphofructokinase by Tetramer-Dimer Conversion.
Tian, T., Wang, C.L., Wu, M.H., Zhang, X., Zang, J.Y.(2018) Biochemistry 57: 4252-4262
- PubMed: 29940104
- DOI: https://doi.org/10.1021/acs.biochem.8b00028
- Primary Citation of Related Structures:
5XOE, 5XZ6, 5XZ7, 5XZ8, 5XZ9, 5XZA - PubMed Abstract:
Most reported bacterial phosphofructokinases (Pfks) are tetramers that exhibit activity allosterically regulated via conformational changes between the R and T states. We report that the Pfk from Staphylococcus aureus NCTC 8325 ( SaPfk) exists as both an active tetramer and an inactive dimer in solution. Multiple effectors, including pH, ADP, ATP, and adenylyl-imidodiphosphate (AMP-PNP), cause equilibrium shifts from the tetramer to dimer, whereas the substrate F6P stabilizes SaPfk tetrameric assembly. Crystal structures of SaPfk in complex with different ligands and biochemical analysis reveal that the flexibility of the Gly150-Leu151 motif in helix α7 plays a role in tetramer-dimer conversion. Thus, we propose a molecular mechanism for allosteric regulation of bacterial Pfk via conversion between the tetramer and dimer in addition to the well-characterized R-state/T-state mechanism.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at Microscale CAS Center for Excellence in Biomacromolecules, Collaborative Innovation Center of Chemistry for Life Sciences, and School of Life Sciences , University of Science and Technology of China , 96 Jinzhai Road , Hefei , Anhui 230026 , China.