Inhibition of homoserine dehydrogenase by formation of a cysteine-NAD covalent complex
Ogata, K., Yajima, Y., Nakamura, S., Kaneko, R., Goto, M., Ohshima, T., Yoshimune, K.(2018) Sci Rep 8: 5749-5749
- PubMed: 29636528
- DOI: https://doi.org/10.1038/s41598-018-24063-1
- Primary Citation of Related Structures:
5X9D - PubMed Abstract:
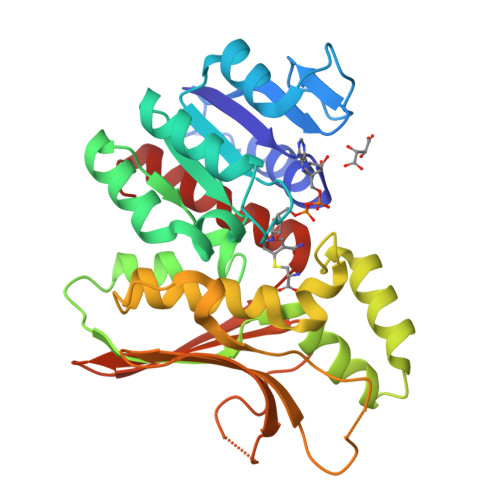
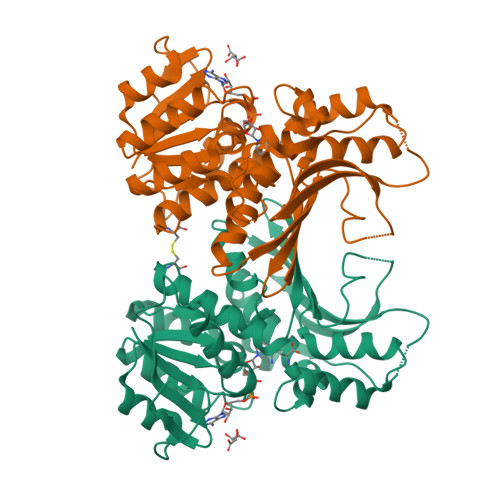
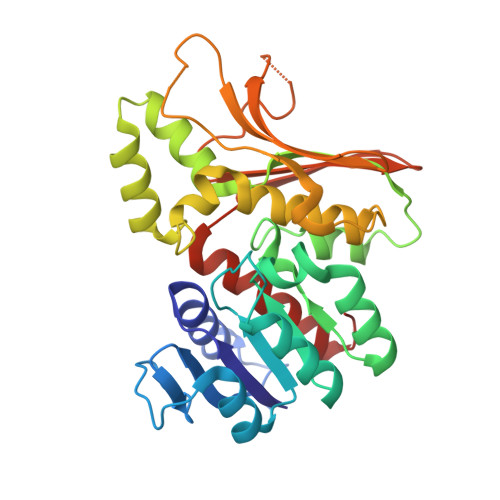
Homoserine dehydrogenase (EC 1.1.1.3, HSD) is an important regulatory enzyme in the aspartate pathway, which mediates synthesis of methionine, threonine and isoleucine from aspartate. Here, HSD from the hyperthermophilic archaeon Sulfolobus tokodaii (StHSD) was found to be inhibited by cysteine, which acted as a competitive inhibitor of homoserine with a Ki of 11 μM and uncompetitive an inhibitor of NAD and NADP with Ki's of 0.55 and 1.2 mM, respectively. Initial velocity and product (NADH) inhibition analyses of homoserine oxidation indicated that StHSD first binds NAD and then homoserine through a sequentially ordered mechanism. This suggests that feedback inhibition of StHSD by cysteine occurs through the formation of an enzyme-NAD-cysteine complex. Structural analysis of StHSD complexed with cysteine and NAD revealed that cysteine situates within the homoserine binding site. The distance between the sulfur atom of cysteine and the C4 atom of the nicotinamide ring was approximately 1.9 Å, close enough to form a covalent bond. The UV absorption-difference spectrum of StHSD with and without cysteine in the presence of NAD, exhibited a peak at 325 nm, which also suggests formation of a covalent bond between cysteine and the nicotinamide ring.
Organizational Affiliation:
Department of Biomolecular Science, Graduate School of Science, Toho University, 2-2-1, Miyama, Funabashi, Chiba, 274-8510, Japan.