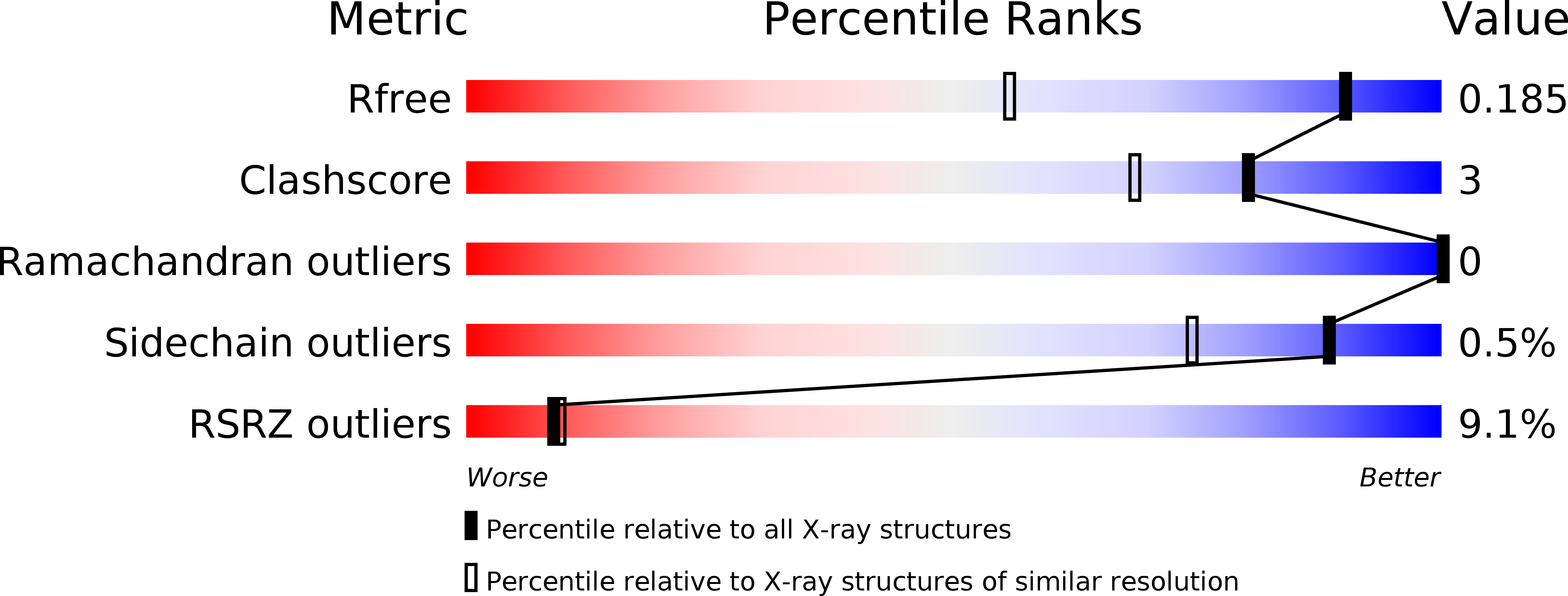
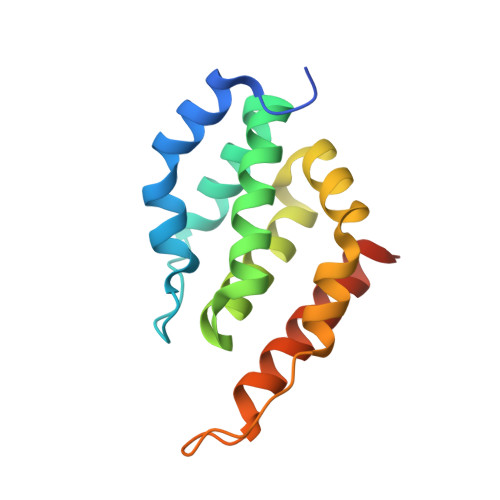
Structure and evolution of ENTH and VHS/ENTH-like domains in tepsin.
Archuleta, T.L., Frazier, M.N., Monken, A.E., Kendall, A.K., Harp, J., McCoy, A.J., Creanza, N., Jackson, L.P.(2017) Traffic 18: 590-603
- PubMed: 28691777
- DOI: https://doi.org/10.1111/tra.12499
- Primary Citation of Related Structures:
5WF1, 5WF2, 5WF9, 5WFB - PubMed Abstract:
Tepsin is currently the only accessory trafficking protein identified in adaptor-related protein 4 (AP4)-coated vesicles originating at the trans-Golgi network (TGN). The molecular basis for interactions between AP4 subunits and motifs in the tepsin C-terminus have been characterized, but the biological role of tepsin remains unknown. We determined X-ray crystal structures of the tepsin epsin N-terminal homology (ENTH) and VHS/ENTH-like domains. Our data reveal unexpected structural features that suggest key functional differences between these and similar domains in other trafficking proteins. The tepsin ENTH domain lacks helix0, helix8 and a lipid binding pocket found in epsin1/2/3. These results explain why tepsin requires AP4 for its membrane recruitment and further suggest ENTH domains cannot be defined solely as lipid binding modules. The VHS domain lacks helix8 and thus contains fewer helices than other VHS domains. Structural data explain biochemical and biophysical evidence that tepsin VHS does not mediate known VHS functions, including recognition of dileucine-based cargo motifs or ubiquitin. Structural comparisons indicate the domains are very similar to each other, and phylogenetic analysis reveals their evolutionary pattern within the domain superfamily. Phylogenetics and comparative genomics further show tepsin within a monophyletic clade that diverged away from epsins early in evolutionary history (~1500 million years ago). Together, these data provide the first detailed molecular view of tepsin and suggest tepsin structure and function diverged away from other epsins. More broadly, these data highlight the challenges inherent in classifying and understanding protein function based only on sequence and structure.
Organizational Affiliation:
Department of Biological Sciences, Vanderbilt University, Nashville, Tennessee.