C. elegans germ granules require both assembly and localized regulators for mRNA repression.
Aoki, S.T., Lynch, T.R., Crittenden, S.L., Bingman, C.A., Wickens, M., Kimble, J.(2021) Nat Commun 12: 996-996
- PubMed: 33579952
- DOI: https://doi.org/10.1038/s41467-021-21278-1
- Primary Citation of Related Structures:
5W4A, 5W4D - PubMed Abstract:
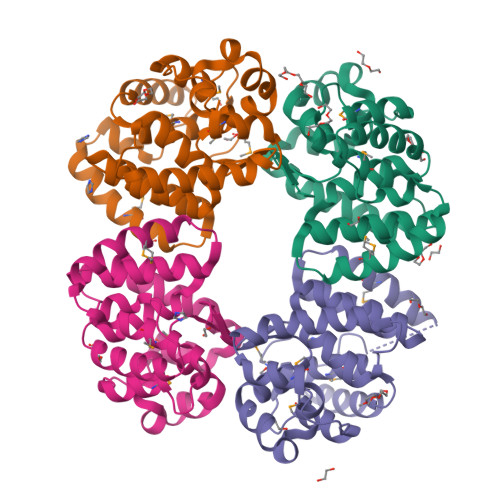
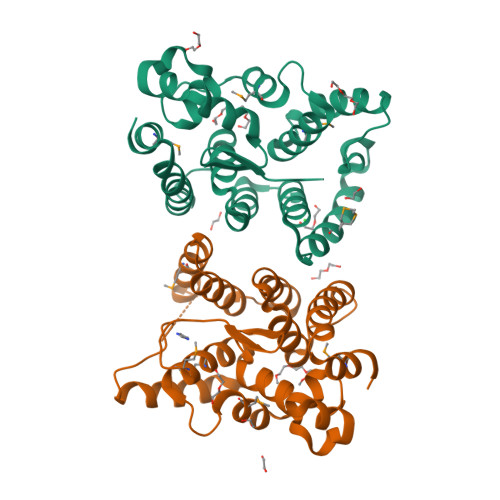
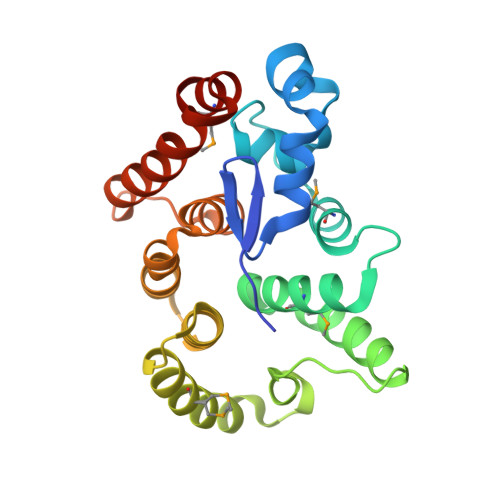
Cytoplasmic RNA-protein (RNP) granules have diverse biophysical properties, from liquid to solid, and play enigmatic roles in RNA metabolism. Nematode P granules are paradigmatic liquid droplet granules and central to germ cell development. Here we analyze a key P granule scaffolding protein, PGL-1, to investigate the functional relationship between P granule assembly and function. Using a protein-RNA tethering assay, we find that reporter mRNA expression is repressed when recruited to PGL-1. We determine the crystal structure of the PGL-1 N-terminal region to 1.5 Å, discover its dimerization, and identify key residues at the dimer interface. Mutations of those interface residues prevent P granule assembly in vivo, de-repress PGL-1 tethered mRNA, and reduce fertility. Therefore, PGL-1 dimerization lies at the heart of both P granule assembly and function. Finally, we identify the P granule-associated Argonaute WAGO-1 as crucial for repression of PGL-1 tethered mRNA. We conclude that P granule function requires both assembly and localized regulators.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, School of Medicine, Indiana University, Indianapolis, IN, USA. staoki@iu.edu.